Progress in mechanism of melatonin regulation of root growth and rhizosphere interactions
-
摘要:目的
根系生长和根际互作是影响植物对土壤养分吸收的关键因子。根系在土壤中穿插生长,不断改变其形态可塑性,进而改变根系构型,扩大与土壤的接触面积以获取所需养分。同时根系的生理可塑性协同根系形态可塑性显著影响根际互作效应,为植物经济高效获取养分资源提供可能。探究褪黑素等内源生长调节因子对根系形态和生理可塑性的调控机制,揭示通过最大化根际效应强化根际互作的有效途径,对集约化作物体系提高养分利用效率,促进绿色增产增效,具有重要的理论与实践意义。
主要进展褪黑素作为新型植物生长调节信号分子,在盐害、干旱和低温等非生物胁迫中具有增强植物抗逆性、改善植物生长等重要调节作用。褪黑素显著改变根系生长,对植物主根生长主要表现为抑制作用,对侧根及不定根的发育和生长具有浓度依赖性调节,从而深刻影响植物根系构型。褪黑素调控根系生长的机制尚不清楚,总结已有进展表明:一方面褪黑素调节光周期,影响光合产物的运输和糖信号,从而调控地下部碳分配和根系生长;另一方面,褪黑素还能与生长素等植物激素互作,参与激素对植物生长调控的信号通路,从而对植物的生长发育和新陈代谢产生影响。这些进展对深入揭示褪黑素调控根系生长发育的机制提供了重要依据。
问题与展望根系的生长发育以及根系构型的改变显著影响根际过程和根际互作,褪黑素作为调控因子在不同养分环境条件下显著影响根系的形态可塑性。然而,褪黑素在根际过程和根际互作中的作用机制并不清楚,有关研究亟待加强。深入探究褪黑素参与根际互作的机制,理解褪黑素调控根系生长和根际过程的作用途径,可为集约化农业体系下精准调控作物根系生长,强化根际互作,提高养分利用效率提供科学依据。
Abstract:ObjectivesRoot growth and rhizosphere interactions are key factors that affect soil nutrient uptake by plant. Roots can fully exert morphological plasticity, change root configuration and expand the contact area with soil to obtain essential nutrients by interpenetrating growth in the structured soil. Moreover, the physiological plasticity of root system, together with its morphological plasticity, can significantly affect and regulate rhizosphere interactions, which may provide the possibility and potential strategies for plants to obtain soil nutrients more efficiently and effectively. Exploring the possible mechanisms to effectively regulate root morphology and physiological plasticity, maximizing rhizosphere processes and strengthening rhizosphere interactions in intensive agriculture systems are of great theoretical and practical importance to improve crop nutrient use efficiency and increase crop yield in a sustainable way.
Major advancesMelatonin as a new signal molecule of plant growth regulation, shows a positive regulatory effect on plant growth and increases tolerance to salt, drought, low temperature, and other abiotic stresses. Melatonin also has a significant regulatory effect on root growth. Melatonin inhabits plant primary roots, whereas it shows a concentration dependence regulation on the development and growth of lateral roots and adventitious roots, thus indicating a profound impact on the root configuration. Although the mechanism of melatonin regulation of root growth is not clear at present, it is speculated that melatonin affects the transport of photosynthetic products and sugar signals, so as to regulate the distribution of carbon flow and root growth in the soil by regulating photoperiod. Meanwhile, melatonin can also be interoperable with plant hormones such as auxin, and participate in the signaling pathway of hormone regulation of plant growth, which has an effect on plant growth, development and metabolism. These new findings provide an important theoretical basis for further revealing the mechanism of melatonin regulation of root growth and development.
Suggestions and expectationsRoot growth and architecture significantly affect rhizosphere processes and rhizosphere interactions. Melatonin, as a regulatory factor, has a significant effect on root morphological plasticity. However, the roles of melatonin in rhizosphere processes and interactions remain largely unclear, and related research is still relatively lacking. Revealing its mechanisms in rhizosphere interactions and understanding its role in regulating root growth and rhizosphere processes could provide the important scientific basis for precisely regulating crop growth, strengthening the rhizosphere interactions, and thus improving the efficient use of nutrients in the intensive cropping systems.
-
Keywords:
- melatonin /
- root growth /
- auxin /
- rhizosphere interactions /
- endophytic bacteria
-
根系从土壤获取水分和养分的过程中发生一系列形态和生理可塑性变化[1-3],以适应土壤养分和水分的时空异质性、不同生物环境因子以及其它不适宜正常生长的逆境胁迫。植物表现出的高度形态可塑性不仅受外部土壤环境(土壤离子浓度、pH、机械阻力、温度与矿质养分分布等)的影响[4],同时也受光合产物、植物激素以及生长调节因子等植物内源物质的严格调控。
根际是植物与土壤相互作用最剧烈的区域,也是根−土−微生物系统中物质与能量交换的重要场所。根际互作在个体尺度上表现为植物地上部与地下部根系的互作,以及根际多过程的互作;在群体尺度上根际互作则为植物−植物之间根际过程的互作,以及植物−土壤−微生物之间的互作[5]。研究表明,在低氮条件下,特定的类黄酮由寄主植物根分泌到根际,同源的(相容的)根瘤菌感知到类黄酮,并被其激活翻译产生NodD蛋白,从而诱导根瘤菌的定殖[6]。此外,最新研究表明,植物生长调节因子一氧化氮(NO)可能作为特殊信号参与根际生物互作,外源低浓度的NO增强了根际解淀粉芽孢杆菌SQR9形成生物膜的能力,同时SQR9的基因yflM可编码一氧化氮合酶(NOS)用于合成NO[7]。植物特异性分泌的某些激素物质或信号因子能够精准地激活和调控植物与植物互作,以及植物与根际微生物的互作。近年来,褪黑素(Melatonin,N-acetyl-5-methoxytryptamine,N-乙酰-5-甲氧基色胺)作为一种新型植物生长调节因子,受到广泛关注。褪黑素是一种小分子的吲哚化合物,存在于多种生物体中,对植物形态建成具有重要作用,特别是在调控根系发育方面具有显著效果[8]。褪黑素可能参与了植物−微生物的相互作用,然而褪黑素调控植物根系发育,影响根际互作的机制并不清楚。本文系统综述了褪黑素对不同植物根系生长的调控作用,重点讨论了褪黑素对植物主根生长、侧根与不定根发生的影响,以及褪黑素对根系构型和养分吸收的调控作用。进一步探讨了褪黑素调控根系生长和根际互作的机制,指出褪黑素在根际互作中可能发挥重要作用,并对褪黑素作为新的调控途径如何影响根际过程和根际互作进行了深入分析,提出了今后的重点研究方向。
1. 褪黑素主要生物学功能概述
褪黑素是一种兼具亲水性和亲脂性的吲哚色胺类的新型生物调节因子,生理活性高且功能多样。褪黑素最早发现于牛的松果体[9],作为哺乳动物及人体重要的神经激素,可以调控昼夜节律,改善睡眠,影响生殖生理[10-11],同时,褪黑素还可以清除机体内多种形态的自由基,延缓衰老,提高免疫抵抗力[12]。随后细菌、真菌、大型藻类以及高等植物中也被证实存在褪黑素[13-15]。1969年褪黑素在绣球百合的胚乳细胞中被检测出[16],直至1995年不同学者通过应用高效液相色谱和放射免疫法在被子植物中检测出褪黑素的存在,这种物质在植物体内的功能才逐渐被深入研究[17-18]。褪黑素被证实是植物体内目前已知的抗氧化作用最强的内源性自由基清除剂[19],种子、叶、根中含量丰富,此外由于叶绿体和线粒体是植物体内活性氧(ROS)的主要产生部位,因而Tan等提出这两种细胞器可能是褪黑素合成的关键部位[20],并在线粒体中检测了褪黑素的含量,但还没有关键合成基因的亚细胞定位分析加以佐证。
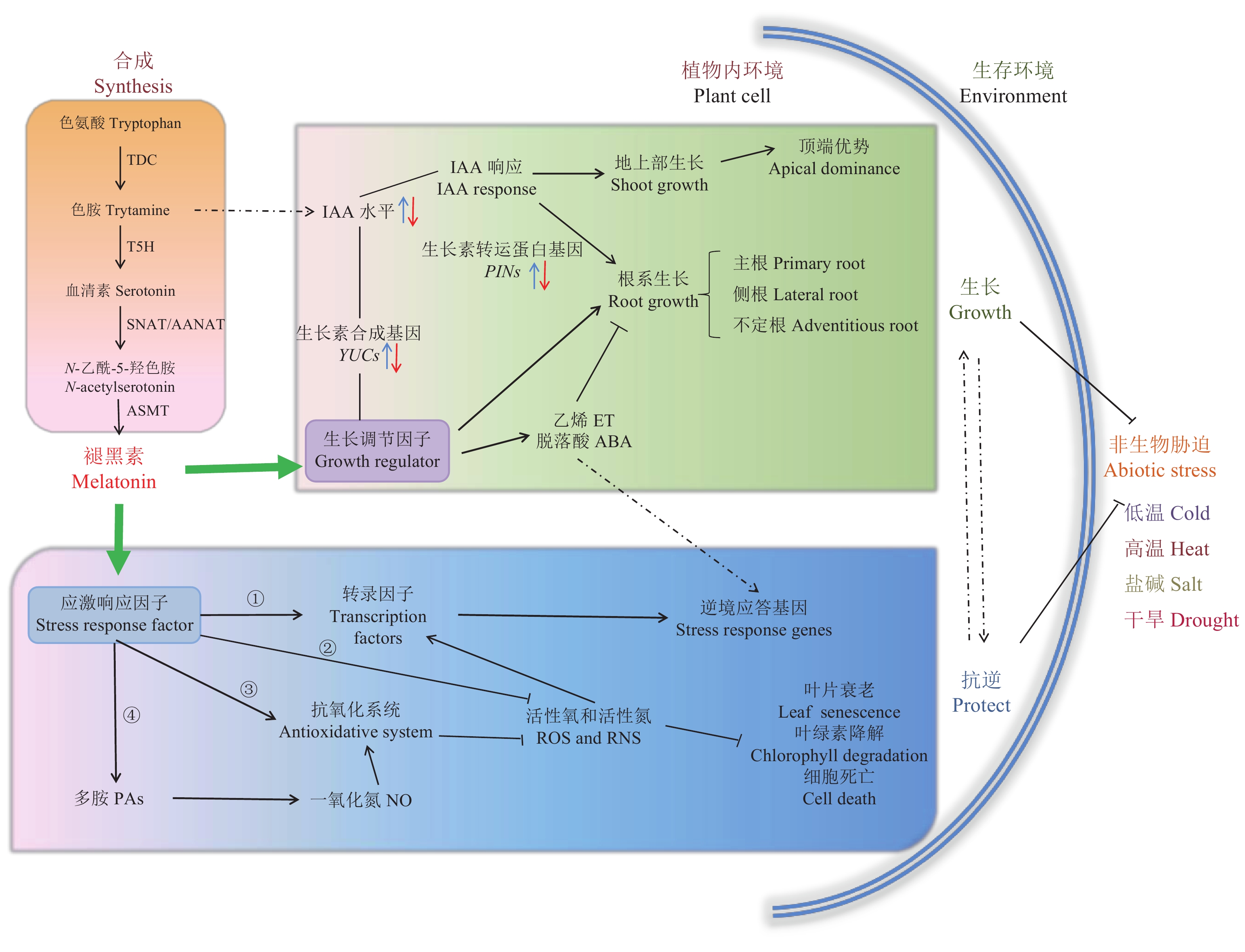
目前褪黑素在植物体内的合成途径较为清晰(图1)。植物体内的褪黑素合成路径与动物体内的合成相似,以色氨酸为底物,由色氨酸脱羧酶(TDC),色胺5-羟化酶(T5H),N-乙酰基转移酶(AANAT)或5-羟色胺N-乙酰转移酶(SNAT),乙酰5-羟色胺甲基转移酶(ASMT)催化的四步连续的酶促反应合成[21-24]。TDC催化色氨酸转化为羟色胺,目前在水稻等植物种类中已克隆出这一关键酶[21,24],但色氨酸脱羧酶合成量低,是合成的限速步骤。T5H催化第二步反应,使色胺转化为褪黑素前体物质血清素,褪黑素生物合成途径的最后两种酶在植物体存在的广泛性仍需进一步验证。褪黑素合成途径中的中间产物色胺也是植物体内生长素(IAA)的合成底物,褪黑素对植物生长发育的调控与IAA可能存在一定的关联,由此推测这可能与二者在植物体内的合成途径有关,相关研究需进一步深化和验证。
随着植物体内褪黑素生物合成等相关研究的逐步深入,褪黑素的生理功能及机理研究已成为国内外学者关注的重点,目前已取得较大进展(图1)。褪黑素作为胁迫条件下应激响应因子被广泛认知。在非生物胁迫条件下,褪黑素能够提高植物的抗性,其调控机制主要表现在以下四个方面:1)褪黑素可直接诱导转录因子调控逆境相关功能基因的表达[25-26],如热激因子(HSFAs)调控热激蛋白基因(HSPs)的表达,从而影响植物的耐热性[27];2)褪黑素是极强的内源性自由基清除物质,高亲脂性使其较易通过生物膜清除细胞内自由基,而其部分亲水性保证其在细胞核中发挥抗氧化作用[19];3)褪黑素作为抗氧化物质,能显著提高植株体内抗坏血酸过氧化物酶(APX)、谷胱甘肽还原酶(GR)等各种与抗氧化胁迫有关的酶活性,增加抗氧化物质含量,从而调节抗坏血酸代谢系统的运转,抑制活性氧的产生,提高植物的抗逆性[28-30];4)在植物受到环境刺激时,褪黑素可诱导植物细胞产生多胺(PAs)和NO,PAs是植物体内调控抗逆性的主要激素,以NO为下游信号诱导植物体内抗氧化系统,从而抵抗非生物胁迫[31-32]。
![]() 图 1 褪黑素在植物中的生物合成途径与生物学功能[注(Note):橘色方框展示褪黑素在植物体内的合成途径The orange box shows the pathway of melatonin synthesis in plants; 蓝色方框展示褪黑素调控植物抗性的途径The blue box shows the pathway of melatonin regulation of plant resistance; 绿色方框展示褪黑素调控植物生长的途径The green box shows the pathway of melatonin regulation of plant growth; 实线表示已有研究支撑的途径,虚线表示推测的可能途径The solid line represents the way to support the existing research, and the dotted line represents the possible way to speculate; 蓝色箭头表示增加,红色箭头表示减少Blue arrows represent increase and red arrows decrease.]Figure 1. Biosynthesis and biological function of melatonin in plants
图 1 褪黑素在植物中的生物合成途径与生物学功能[注(Note):橘色方框展示褪黑素在植物体内的合成途径The orange box shows the pathway of melatonin synthesis in plants; 蓝色方框展示褪黑素调控植物抗性的途径The blue box shows the pathway of melatonin regulation of plant resistance; 绿色方框展示褪黑素调控植物生长的途径The green box shows the pathway of melatonin regulation of plant growth; 实线表示已有研究支撑的途径,虚线表示推测的可能途径The solid line represents the way to support the existing research, and the dotted line represents the possible way to speculate; 蓝色箭头表示增加,红色箭头表示减少Blue arrows represent increase and red arrows decrease.]Figure 1. Biosynthesis and biological function of melatonin in plants另一方面,褪黑素作为植物体内的生长调节物质[27、33-35],调控许多植物的生长发育特征,包括种子萌发、幼苗生长、开花结实和衰老等[36-38]。研究表明,外源添加0.5~1.0 μmol/L的褪黑素,增加了水稻种子根的长度以及根的生物量[39];向土壤中长期施用100 μmol/L褪黑素能够改变苹果的新陈代谢水平并且维持其蛋白质活性,提高光合速率,增加光合产物[40]。有关褪黑素调控植物根系生长的研究,目前表观描述性的研究较多,但揭示分子机制的研究较为缺乏。褪黑素作为影响植物根系的重要调控因子,如何参与根际互作,从而影响养分效率,尚无系统性报道。
2. 褪黑素对根系生长的影响
根系是植物长期适应陆地环境进化而来的营养器官,既帮助植物从土壤中吸收水分和养分,又将植物固定在土壤上[41]。根系通常包括主根与侧根,根系的构型和空间扩展主要依赖于侧根的生长,侧根通常起源于原生木质部的中柱鞘细胞。根系具有吸收、运输养分和水分的功能,从而保证植物的地上部生长。除此之外,有些植物的茎或叶在感受外界环境刺激或者在激素的诱导下,可产生不定根。侧根和不定根的发育体现了根系的高度可塑性,一定程度上受植物体内激素的精确调控[42-43]。外源施用IAA或提高体内IAA浓度会增加不定根和侧根的形成,而生长素转运抑制剂则具有显著的抑制效果[44-45]。研究表明,莴苣外源施用6-苄基氨基嘌呤(BA)可使侧根减少50%[46],转基因烟草过表达拟南芥细胞分裂素氧化酶基因(CKX),植物侧根数量显著增加[47]。这些被人们熟知的生长素、细胞分裂素(CTK)等激素对植物根系生长发育具有重要调节作用,调控途径也较为清楚。而褪黑素作为一种新型的植物内源调节物质,其对植物生长的调节作用被广泛报道,特别是对根系生长具有明显的调节作用,研究表明这种调节作用与褪黑素的浓度、植物种类、根系类型以及环境胁迫条件密切相关[48-50]。
2.1 褪黑素对植物主根和种子根的影响
褪黑素显著影响单子叶植物主根的生长。Hernández-Ruiz等[48]用不同浓度梯度的外源褪黑素处理金丝雀草、小麦、燕麦三种单子叶植物48小时,结果显示不同单子叶植物主根对褪黑素刺激的响应存在差异。与对照相比,不同浓度梯度的褪黑素均显著抑制金丝雀草主根的生长,并随浓度增加,抑制效果增加;燕麦主根受褪黑素影响趋势与金丝雀草相同,但被抑制程度更大;而褪黑素对小麦主根的调控具有双峰效应,即低浓度褪黑素显著促进主根生长,而高浓度表现出抑制效果。褪黑素刺激效果不同的原因可能是植物对褪黑素的代谢能力存在差异,导致植物体内其积累量不同。转基因水稻(Oryza sativa)体内过表达绵羊5-羟色胺N-乙酰基转移酶,在幼苗生长4天时,与野生型相比种子根根长平均增加了82%,第10天时增长了75%,同时测得转基因水稻内源褪黑素水平显著高于野生型[39]。尽管褪黑素对主根的调控效果因植物种类不同,但相关研究报道证明了褪黑素在单子叶植物胚根发育中起着关键作用。
大多数双子叶植物根系为直根系,主根发达,在土壤中下扎较深[51]。褪黑素对双子叶植物主根生长的影响与单子叶植物相似,主要表现为抑制作用。模式植物拟南芥(Arabidopsis thaliana)在含有不同浓度的褪黑素培养基上生长6天,高浓度褪黑素显著抑制主根生长,而低浓度褪黑素则无明显效果,褪黑素主要是通过减少分生组织细胞数量对主根的生长产生抑制作用[52]。不同植物生育时期对褪黑素的响应存在差异,研究发现2日龄的野生荠菜黄化苗在0.1 μmol/L褪黑素处理下主根显著增长,而100μmol/L褪黑素则抑制其生长;但4日龄的幼苗对低浓度褪黑素无响应,仅在高浓度褪黑素处理下表现出主根生长受阻[49]。综上所述,褪黑素对植物主根的影响以抑制作用为主,少数植物的主根对其并不敏感,这主要与作物种类和褪黑素作用浓度有关。
2.2 褪黑素对植物侧根和不定根的影响
褪黑素对不同植物侧根和不定根的生长发育具有显著的调控作用。侧根与不定根的发生与伸长,增加了与土壤接触的面积,根分泌物可影响土壤养分的活化,提高作物对土壤养分资源的捕获和利用能力,满足植物地上部快速生长对水分和矿质养分的需求,这是植物提高养分和水分空间有效性与活化利用效率的重要生物学机制[53-55]。Arnao和Hernández-Ruiz[56]用不同浓度褪黑素处理羽扇豆下胚轴,以探究褪黑素对植物不定根和侧根发育的影响,结果表明,褪黑素能诱导侧根原基的出现,改变不定根和侧根的分布格局与时间进程,以及不定根数量和长度。进一步的研究证明褪黑素能促进多种植物的侧根和不定根的生长发育,在拟南芥中,褪黑素显著增加不定根的发生,与对照相比侧根发生提高了三倍,但对根毛密度影响不显著[57]。在过表达褪黑素合成基因的三个拟南芥转基因株系中,与野生型相比侧根大量增生[58]。
除了以上模式作物以外,Zhang等研究发现水分胁迫下,黄瓜(Cucumissativus L.)侧根生长受到显著抑制,而褪黑素处理可以缓解这一抑制作用,诱导侧根数量增加1.25倍[59]。进一步的研究表明黄瓜在盐胁迫下,褪黑素处理下调MYB、WRKY、NAC等转录因子,而这些转录因子对侧根生长具有负向调控作用。促进不定根发生的现象也在番茄(SolanumlycopersicumL.)中被发现[60]。褪黑素对于商业甜樱桃[61]、石榴[62]等果树的砧木或外植体也具有促进不定根发生的作用,作为园艺栽培手段,褪黑素在园艺上具有巨大的经济应用价值。总之,适宜浓度的褪黑素处理能显著促进植物侧根和不定根的生长,有利于植物对养分的吸收和抗胁迫能力的提高。
2.3 褪黑素对植物根系构型的影响
在长期生长进化中植物形成了复杂的三维根系构型,由分支、表面积、角度等参数决定的根系构型很大程度上影响了根系探索土壤空间的能力,因此对根系构型的调节显著影响植物对养分获取和吸收利用的效率[63]。根系在生长介质的延伸以及空间分布不仅受外界环境(养分分布、土壤结构和土壤水分等)的影响,同时植物根系表现出的高度可塑性也受内源信号的主动调节[64]。褪黑素不仅能调控多种植物不同类型根系的发育生长,同时已有研究也表明褪黑素显著影响根系构型。褪黑素可刺激侧根和不定根形成,从而改变根系构型。50 μmol/L褪黑素处理可造成直根系植物苜蓿体内过氧化氢(H2O2)的积累,刺激细胞周期调控基因MsCDKB1;1和MsCDKB2;1的显着表达,从而促进侧根大量增生[65]。须根系水稻在褪黑素处理下,侧根和节根的数量和长度显著增加而主根生长受到抑制[66]。此外,褪黑素还能影响根的向地性,研究表明,羽扇豆初生根受到100 μmol/L褪黑素刺激时,根的向地性丧失[67]。
作物生长取决于对水和矿物质的获取能力,根系生长以及根系构型的改变显著影响作物获取养分的效率[4]。研究发现,0.1 mmol/L褪黑素显著缓解了黄瓜由于高硝酸盐水平(0.6 mol/L)对植物生长的抑制作用,同时氮代谢系统中调节硝酸还原酶和谷氨酸合酶活性的两个基因Cs-NR和Cs-GOGAT的表达均显著上调。硝酸盐胁迫下,褪黑素可以调控根系生长,促进侧根增生,增加根冠比,从而调节植株体内磷、钾、铁、镁等矿质养分的浓度,改善作物生长[68]。对苹果的研究表明,褪黑素显著提高盐胁迫和钾胁迫下根系和叶子的钾含量,激活钙调磷酸酶类蛋白及其互作蛋白激酶形成的CBL1-CIPK23通路,调节钾通道蛋白基因的表达,从而促进钾离子的吸收[69]。因此,褪黑素可以通过调控根系构型和养分吸收对植物生长产生促进作用。
3. 褪黑素调控根系生长的可能机制
同一基因型的植物在不同的环境条件下根系构型可能发生巨大的变化。褪黑素对植物根系生长具有显著调节作用,然而已有研究对调控过程的内在机制并没有系统性报道。大量研究指出根系发育和建成需要光合碳的投入,光合作用为植物体提供了物质和能量来源,因而光合作用的改变以及有机产物特别是糖信号的变化显著影响根系的生长和发育[70]。同时,激素在胚根的早期建立及胚后根的生长中都起到重要的调控作用,不同激素信号之间存在着复杂的相互作用,赋予了根系生长的可塑性和灵活性[71]。因此,褪黑素可能参与调控植物体内光合作用,刺激糖信号改变,同时与植物激素网络产生互作,进而调控植物根系的生长。
3.1 褪黑素通过调控光合作用和糖信号影响根系生长
植物根系大量增生的过程是光合产物碳输入和消耗的过程,碳水化合物为根系生长提供了物质和能量来源[51]。研究表明,根干重增加1 g需要消耗2 g碳水化合物[72],随着植物不断生长,根系增生需要更多的光合碳输入,植物每天光合产物的25%~50%运输到根系用于根系新生组织生长以及维持根系正常生长代谢[73-74]。因此当植物的光合作用、光合产物运输和分配以及根系对碳的利用发生改变时,根系生长必然受到影响[75]。
第一,褪黑素可能参与光周期调控,从宏观时间尺度上影响不同植物根系的生长速率。昼夜周期中光照和黑暗期长短的交替变化不仅调控花芽分化、开花、结实,而且是植物根系生长的重要调节器[76-80]。褪黑素在某些植物种类中的生物合成具有昼夜节律,暗示着植物光周期可能受这种物质的调节[81]。一些植物的细根生长表现出明显的24小时节律,在固定的14小时光周期下,根系生长速率随昼夜节律变化明显,最大根生长速率发生在黑暗期来临前后的2小时。随后,在黑暗时期的后期,光周期的前期,根系生长速率下降并达到最小值,然而调节根系速率昼夜周期变化的内源因素尚不清楚[82]。目前研究显示褪黑素可能作为植物昼夜节律的重要调节因子,而另一方面它也表现出促进某些植物侧根伸长的作用,推测褪黑素影响根系生长的机制可能与调控昼夜节律密切相关,但仍缺乏实证研究和深入探讨。
第二,褪黑素能够保护光合色素(叶绿素和类胡萝卜素),增强光系统的光合效率,增加了CO2同化速率和气孔导度,促进光合作用产生更多的物质和能量[33,40, 83],为保持植物根系生长提供物质条件。冷胁迫使水稻叶绿素和类胡萝卜素含量降低,褪黑素预处理显著增加幼苗叶绿素和类胡萝卜素含量,缓解冷胁迫对植物生长的抑制作用,同时提高净光合速率、气孔导度和细胞间CO2浓度[84]。低浓度褪黑素处理栽培作物樱桃砧木,显著增加了根长,特别是1 μmol/L浓度处理使根长增加2.5倍,并且根的鲜重增加4倍,测定叶绿素含量结果也显示低浓度褪黑素显著增加作物叶绿素总量[50]。研究表明,外源褪黑素处理的低等植物轮藻,其光系统Ⅱ光化学量子产率提高了34%,这可能是由于光系统Ⅱ的开放反应中心数量的增加,推测其原因是褪黑素具有良好的抗氧化性,保护了叶绿素和光合蛋白,从而保证了所有光合传递链组分发挥更好的功能[85]。褪黑素促进光合作用的进行,充分保证了植物体物质和能量的供应,为褪黑素调控下根系生长和根系构型改变所需碳源和能源提供了重要的物质条件。
第三,褪黑素能够影响光合产物的合成与代谢,调控糖信号,干扰光合产物在韧皮部装载及分配,从而影响源库关系来调控植物根系生长。植物能够通过协调营养物质水平和自身生长需求,从而确保特定生理过程所需物质和能量的充足供应[86]。大量研究表明,可溶性糖能作为信号调控植株体内基因表达从而影响植物发育,对植物生长和形态发育产生显著影响[87];糖信号可以刺激并影响蛋白激酶活性,与脱落酸等植物激素相互作用调控植物早期的胚胎发育、种子萌发和幼苗生长[88-90];同时糖信号还可以参与调控叶、结节、花粉、块茎等组织结构的建成[91-92];此外,糖信号对根系发育的调控也已有大量报道。当野生型拟南芥幼苗在含有0.5%~2.0%蔗糖的培养基中于黑暗下生长时,下胚轴上不定根的形成受到刺激[70];低磷胁迫下,白羽扇豆缺磷诱导的基因需韧皮部运输的糖信号参与调控,从而显著影响排根的形成和根际过程[93]。
褪黑素能够上调参与蔗糖合成的蔗糖磷酸合成酶(SPS)和蔗糖合成酶(SUS)两种酶的基因表达,特别是100 μmol/L褪黑素的上调作用显著,同时降低蔗糖转化酶(INV)抑制基因INVINH1和INVINH4的表达,提高转化酶活性,从而促进蔗糖水解转化[94]。在拟南芥中,外源施用50 μmol/L褪黑素下调INVINH相关基因的表达,上调转化酶活性[95]。更重要的是研究发现高浓度褪黑素下调叶片蔗糖共质体运输相关基因Tdy2和Sxd1,阻断了蔗糖共质体转运,诱导了玉米叶片源部分淀粉和蔗糖的积累,而蔗糖的积累既显著降低了光合速率[95-96],又影响了光合产物在源与库之间的运输与协调,这也是褪黑素影响光合产物运输与分配,从而调控根系的重要机制之一。
3.2 褪黑素通过调控植物激素影响根系生长
植物根系的生长发育受植物激素的严格调控,生长素、细胞分裂素、乙烯(ETH)等调控侧根发生、根细胞伸长、根毛形成以及根向地性等过程[42-43]。生长素作为最早发现的植物激素被认为是对根系生长产生关键调控作用的激素。在茎尖和幼叶中合成的生长素通过极性运输进入根系,调控相关基因表达,诱导侧根原基的发生[97]。研究表明褪黑素在调节植物生长方面表现出与IAA相似的作用[48,98],褪黑素与IAA都是吲哚化合物,褪黑素是由色氨酸脱羧成色胺经一系列酶促反应生成[21],而生长素的合成途径之一也是色胺合成途径[99]。因此,深入探究褪黑素对根系生长的调控与IAA信号途径的关系,对于理解褪黑素介导的根系形态与构型的变化至关重要。
高浓度褪黑素通过减少根分生组织显著抑制拟南芥主根长度,试验证明600 μmol/L褪黑素显著下调与IAA合成相关的基因Yucca(YUC1、YUC2、YUC5、YUC6)的转录水平,而另一部分(YUC3、YUC4、YUC7、YUC8)上调,同时下调IAA转运蛋白的表达[52]。在水稻上得到了类似的结果,褪黑素处理显著抑制胚根的生长,促进侧根的形成和发育。全基因组RNA测序分析结果表明,褪黑素使生长素相关的转录因子、根系生长和发育相关候选顺时元件以及生长素相关过程的基因均过量表达[66]。这些研究表明褪黑素调控根系发育的途径可能与生长素通路发生互作,但具体的关键基因和作用方式并没有明确的报道。然而,褪黑素并不能直接激活拟南芥中生长素报告基因DR5∶GUS的表达 [57]。虽然褪黑素与生长素均含有吲哚环,C3位置有侧链,但生长素活性的结构要求是不饱和环、酸性侧链,以及特殊的空间构型[99],而褪黑素结构的侧链不具有酸性且空间构型存在差异。因此,褪黑素不满足生长素活性的结构要求,不能直接作为生长素的替代物质发生作用。此外黄瓜的研究表明,褪黑素处理直接显著影响了根系生长的相关基因[59]。因而褪黑素对根系发育的调控途径之一可能是与生长素调控途径偶联,另一方面褪黑素调控根系生长也可激活独立于生长素之外的调控通路。
调控根系生长的重要激素还有细胞分裂素,其直接参与了细胞周期的控制和调节,同时对分生组织的发育起至关重要的作用[99]。对于根系生长而言,细胞分裂素和生长素表现出拮抗作用;生长素促进侧根和不定根的形成[100-102],而细胞分裂素效果相反,抑制侧根形成[46]。目前研究显示褪黑素调节根系生长也可能独立于生长素,而细胞分裂素可能作为褪黑素调节的另一个信号通路,影响根系生长和发育,但相关研究极为缺乏。除此之外,乙烯可以通过与细胞分裂素互作抑制根系生长[103],同时通过影响生长素生物合成和运输,改变生长素分布,调节根系生长[104]。研究表明褪黑素处理能够负向调控与乙烯相关的转录因子,以及与生根相关的基因,从而抑制根的发生与伸长[105]。对于褪黑素与其他植物激素互作调控根系生长的研究报道相对较少,有关方面需要进一步的深入研究。除了植物激素以外,NO是植物体内重要的信号分子,NO参与了调节根系生长,影响不定根、侧根和根毛形成中的生长素应答[106-107]。番茄幼苗的试验表明,褪黑素诱导的不定根发生与NO介导的下游信号有关。褪黑素可上调硝酸还原酶,增加内源性NO水平,同时NO也可反馈调节植物体内褪黑素水平[60]。
4. 褪黑素影响根际互作的可能途径
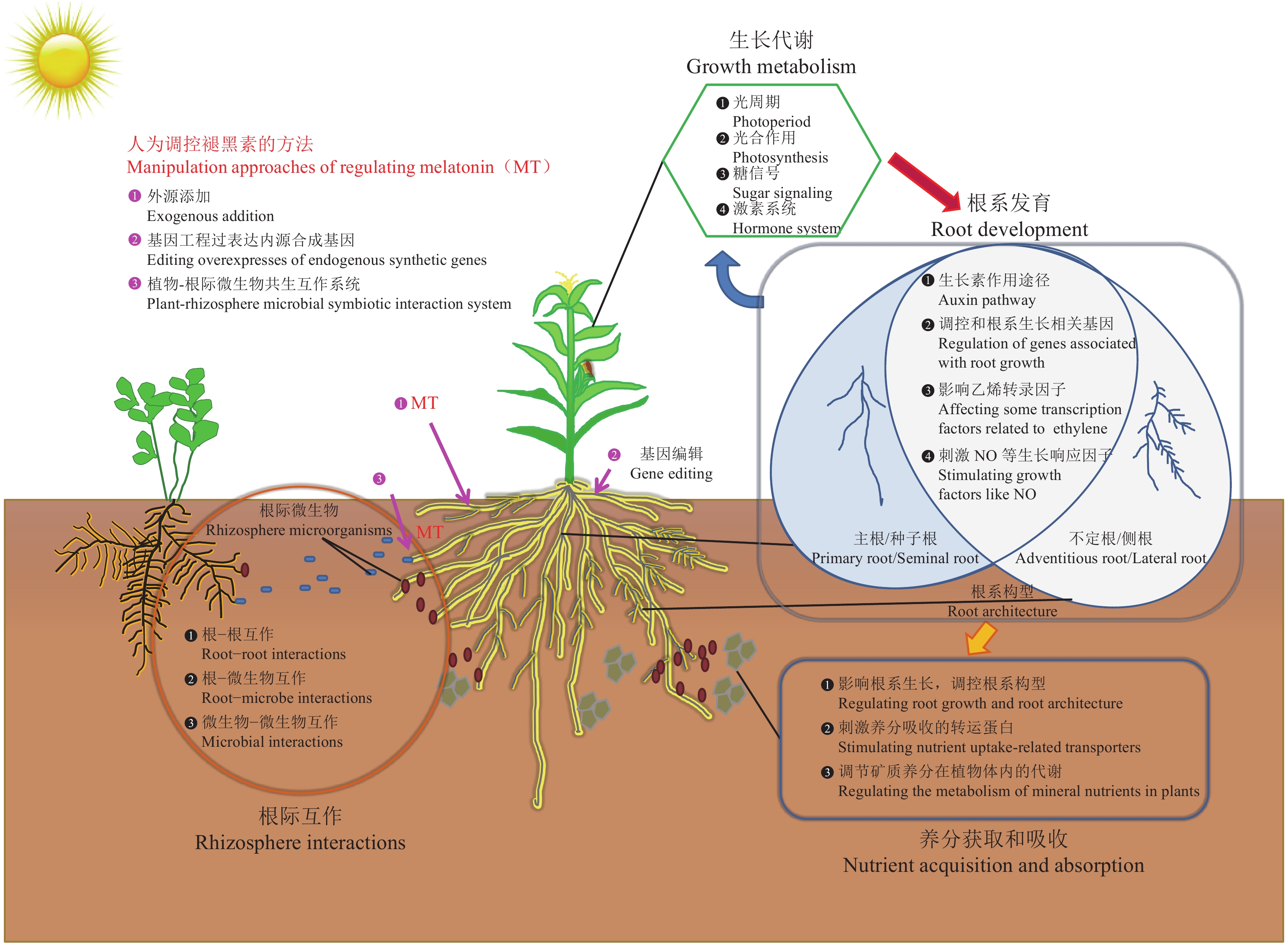
植物根系生长于复杂的土壤环境中,从突破种皮开始生长便与土壤发生直接的相互作用。根系可以感受到自然土壤的结构和资源时空分布不均匀,做出一系列形态与生理可塑性改变以适应土壤环境。干旱的土壤对根系的水分供应能力较弱,而且阻碍根系穿插,显著抑制植物根系的生长。而抗旱植物可形成深根系以获取下层土壤中的水分,保证自身生长[108]。遇到营养富集的土壤斑块时,许多植物会增加根系的分枝和生长,充分吸收养分以满足生长需求[109]。羽扇豆、蚕豆等豆科植物遇到养分匮乏的土壤环境可增加根系质子、有机酸和酸性磷酸酶的分泌,活化土壤中的难溶性Fe/Al-P以及有机磷,提高土壤有效磷含量,促进养分吸收和植物生长[41]。根−土互作过程不仅表现在土壤结构和养分资源对植物根系形态和生理可塑性的影响,植物根系也可改变和重塑土壤环境,从而强化根−土互作过程。根系通过下扎、水分吸收、根分泌物释放等方式显著改变根际土壤结构。植物具有较大的根生物量,可以增加土壤大孔隙(裂缝)的表面积和长度,粗根可以使大孔隙增加30%。根系生长和根构型变化也会影响土壤−水的关系,根系吸水不均匀产生的拉伸应力会导致土壤团聚体的产生,同时使土壤更致密,抗拉强度更高[110]。植物根系还可以通过根分泌物释放从宏观时间尺度上影响土壤有机质含量[111]。褪黑素调控的根系增生和根构型变化可能从多方面影响原有状态下植物根−土互作:1)增大了与土壤的接触面积,即根−土界面的范围进一步扩大,根系与土壤养分直接接触的几率增加,根际过程的强度显著提升;2)破坏和改变了原有的土壤环境,土壤孔隙度、紧实度、含水量、有机质含量等土壤结构,与养分空间分布发生巨大变化,对不同生育时期的植物生长造成一定影响。
生态系统内植物在不同尺度上形成种群或群落,由于对土壤空间和资源的需求,根−根相互作用必然发生。种内互作是同种植物间根系受密度和种群内部对土壤资源获取的影响而发生的相互作用,种间互作则为不同植物间产生的互作效应。互作体系下的植物根系产生可塑性变化以及调整根系构型,通过躲避(avoidance)、耐受(no responsive pattern)、入侵(aggregation)对相邻植物作出响应[112],保证自身对土壤空间的占据及资源的获取。土壤资源和根系的空间匹配受互作体系中个体植株根系构型的影响,褪黑素作为根系构型的内源调节物质可能对植物间的根系互作产生直接影响;更重要的是,植物在获取不同空间范围土壤养分时,褪黑素对植物根系可塑性的调控可能影响根系的水平扩展,避免植物对有限资源的直接竞争,进而提高整个系统的生产力。
植物与土壤微生物之间密切的相互作用是根际互作的重要组成部分。植物根系通过分泌多种初级代谢产物(有机酸、碳水化合物和氨基酸等)和次级代谢产物(生物碱、萜类化合物和酚类等),以信号、物质流或某种形式影响根际微生物群落,招募和促进有益微生物的定殖和生长,同时抵抗病原性或其他有害微生物[113]。目前已知的多种植物内源生长调节物质如黄酮类化合物、独脚金内酯等都是植物−微生物互作的关键信号[5]。同时,根际微生物不仅能感知植物或其他微生物传递的信号,还能够特异性释放多种信号分子影响植物宿主。微生物分泌的脂多糖、肽聚糖、鞭毛蛋白、甲壳素[114-116]等触发植物响应,而后由相关信号通过植物激素水杨酸、茉莉酸、乙烯和其他调控网络,控制免疫防御与共生系统的建立[116-119]。微生物还可通过与根系的直接养分交换或者自身代谢分泌物影响根系生长[120]。Jiao等从三个葡萄品种中分离并鉴定根内生菌解淀粉芽孢杆菌SB-9(Bacillus amyloliquefaciens SB-9) ,在体外表现出较高水平的褪黑素分泌能力,接种后促进葡萄植株的生长。此外,在暴露于盐胁迫或干旱胁迫的葡萄苗中,解淀粉芽孢杆菌SB-9的定殖增加了葡萄苗体内褪黑素合成以及其中间体的上调[121]。这一研究为有益内生菌和寄主植物通过褪黑素进行的根际互作提供了有利证据。另一种葡萄内生菌荧光假单胞菌RG11 (Pseudomonas fluorescens RG11)能够促进植株生长,并提高不同葡萄品种内源性褪黑素的水平,同时在四个葡萄品种中发现了褪黑素前体物质含量的种内变化,相关的根粗提物也显示RG11可能诱导褪黑素的体外生物合成[122]。这一研究提出了根际细菌中潜在的褪黑素合成途径,并揭示了褪黑素生物合成的保守性以及植物−根际细菌互作体系下的褪黑素合成网络。褪黑素是否会作为根际信号物质调控根际微生物在植物中的定殖还是一个未知问题,它是否介导其他植物−微生物共生互作还有待进一步研究(图2)。
5. 结论与展望
褪黑素作为植物体内的小分子信号物质,其生理功能与作用机制正得到逐步阐释。本文系统总结了褪黑素对植物主根、侧根以及根系构型的调控作用,探讨了可能的调控机制与途径:一是通过影响地上部光合作用以及糖信号,从而调控根系生长所需的物质和能量输入;二是通过与生长素、乙烯等植物激素以及NO等信号分子互作,参与不同生长信号网络的调控过程。褪黑素对根系生长的调控进一步影响了根土界面养分和水分的获取,从而影响植物对养分的利用效率。植物根系生长和根系构型的变化,可显著影响植物种内与种间的根−根互作、根际互作(间作、轮作)以及根−土−微生物之间的互作。褪黑素可能作为关键调控因子影响多种根际过程,其对根系生长和根际互作的影响将是未来深入研究根际互作调控机制的重点和方向。
-
图 1 褪黑素在植物中的生物合成途径与生物学功能
[注(Note):橘色方框展示褪黑素在植物体内的合成途径The orange box shows the pathway of melatonin synthesis in plants; 蓝色方框展示褪黑素调控植物抗性的途径The blue box shows the pathway of melatonin regulation of plant resistance; 绿色方框展示褪黑素调控植物生长的途径The green box shows the pathway of melatonin regulation of plant growth; 实线表示已有研究支撑的途径,虚线表示推测的可能途径The solid line represents the way to support the existing research, and the dotted line represents the possible way to speculate; 蓝色箭头表示增加,红色箭头表示减少Blue arrows represent increase and red arrows decrease.]
Figure 1. Biosynthesis and biological function of melatonin in plants
-
[1] Jackson R B, Caldwell M M. Integrating resource heterogeneity and plant plasticity: modeling nitrate and phosphate uptake in a patchy soil environment [J]. Journal of Ecology, 1996, 84(6): 891–903. DOI: 10.2307/2960560
[2] Gross K L, Peters A, Pregitzer K S. Fine root growth and demographic responses to nutrient patches in four old–field plant species[J]. Oecologia, 1993, 95(1): 61–64. DOI: 10.1007/BF00649507
[3] Robinson D. The responses of plants to nonuniform supplies of nutrients[J]. New Phytologist, 1994, 127(4): 635–674. DOI: 10.1111/nph.1994.127.issue-4
[4] Jin K, White P J, Whalley W R, et al. Shaping an optimal soil by root-soil interaction[J]. Trends in Plant Science, 2017, 22(10): 823–829. DOI: 10.1016/j.tplants.2017.07.008
[5] 张福锁, 申建波, 冯固. 根际生态学—过程与调控[M]. 北京: 中国农业大学出版社, 2009. Zhang F S, Shen J B, Feng G. Rhizosphere ecology−process and regulation[M]. Beijing: China Agricultural University Press, 2009.
[6] Liu C W, Murray J D. The role of flavonoids in nodulation host-range specificity: An update[J]. Plants, 2016, 5(3): 33–46. DOI: 10.3390/plants5030033
[7] Dong X, Liu Y, Zhang G, et al. Synthesis and detoxification of nitric oxide in the plant beneficial rhizobacterium Bacillus amyloliquefaciens, SQR9 and its effect on biofilm formation[J]. Biochemical and Biophysical Research Communications, 2018, 503(2): 784–790. DOI: 10.1016/j.bbrc.2018.06.076
[8] Erland L A E, Saxena P K. Melatonin in plant morphogenesis[J]. In Vitro Cellular & Derelopmental Biology-plant, 2018, 54(1): 3-24.
[9] Lerner A B, Case J D, Takahashi Y, et al. Isolation of melatonin, the pineal gland factor that lightens melanocytes[J]. Journal of the American Chemical Society, 1958, 80(10): 2857–2587.
[10] Reiter R J, Melchiorri D, Sewerynek E, et al. A review of the evidence supporting melatonin's role as an antioxidant[J]. Journal of Pineal Research, 1995, 18(1): 1–11.
[11] Venegas C, García J A, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations[J]. Journal of Pineal Research, 2012, 52(2): 217–227. DOI: 10.1111/jpi.2012.52.issue-2
[12] Tan D X, Manchester L C, Hardeland R, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin [J]. Journal of Pineal Research, 2003, 34(1): 75–78. DOI: 10.1034/j.1600-079X.2003.02111.x
[13] Tilden A R, Becker M A, Amma L L, et al. Melatonin production in an aerobic photosynthetic bacterium: an evolutionarily early association with darkness[J]. Journal of Pineal Research, 1997, 22(2): 102–106. DOI: 10.1111/jpi.1997.22.issue-2
[14] Rodriguez-Naranjo M I, Torija M J, Mas A, et al. Production of melatonin by Saccharomyces strains under growth and fermentation conditions[J]. Journal of Pineal Research, 2012, 53(3): 219–224. DOI: 10.1111/jpi.2012.53.issue-3
[15] Tan D X, Hardeland R, Manchester L C, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science [J]. Journal of Experimental Botany, 2012, 63(2): 577–597. DOI: 10.1093/jxb/err256
[16] Jackson W T. Regulation of mitosis.II. Interaction of isopropyl N-phenylcarbamate and melatonin [J]. Journal of Cell Science, 1969, 5(3): 745–755.
[17] Dubbels R, Reiter R J, Klenke E, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry [J]. Journal of Pineal Research, 1995, 18(1): 28–31. DOI: 10.1111/jpi.1995.18.issue-1
[18] Hattori A, Migitaka H, Iigo M, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates [J]. Biochemistry and Molecular Biology International, 1995, 35(3): 627–634.
[19] Reiter R J, Guerrero J M, Escames G, et al. Prophylactic actions of melatonin in oxidative neurotoxicity [J]. Annals of the New York Academy of Sciences, 2010, 825(1): 70–78.
[20] Tan D X,Mnchester L C, Liu X, et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes [J]. Journal of Pineal Research, 2013, 54(2): 127–138. DOI: 10.1111/jpi.2013.54.issue-2
[21] Kang S, Kang K, Lee K, et al. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice [J]. Planta, 2007, 227(1): 263–272. DOI: 10.1007/s00425-007-0614-z
[22] Fujiwara T, Maisonneuve S, Isshiki M, et al. Sekiguchilesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice [J]. Journal of Biological Chemistry, 2010, 285(15): 11308–11313. DOI: 10.1074/jbc.M109.091371
[23] Byeon Y, Lee H Y, Lee K, et al. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT[J]. Journal of Pineal Research, 2014, 56(1): 107-114. DOI: 10.1111/jpi.2013.56.issue-1
[24] Okazaki M, Higuchi K, Hanawa Y, et al. Cloning and characterization of a Chlamydomonas reinhardtii cDNA arylalkylamine N-acetyl transferase and its use in the genetic engineering of melatonin content in the Micro-Tom tomato[J]. Journal of Pineal Research, 2009, 46(4): 373–382. DOI: 10.1111/jpi.2009.46.issue-4
[25] Bajwa V S, Shukla M R, Sherif S M, et al. Role of melatonin in alleviating cold stress in Arabidopsis thaliana [J]. Journal of Pineal Research, 2014, 56(3): 238–245. DOI: 10.1111/jpi.12115
[26] Liu N, Gong B, Jin Z, et al. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal [J]. Journal of Plant Physiology, 2015, 186–187: 68–77.
[27] Shi H, Tan D X, Reiter R J, et al. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis [J]. Journal of Pineal Research, 2015, 58(3): 335–342. DOI: 10.1111/jpi.2015.58.issue-3
[28] Russel J R P D, Dun X T M D, Susanne B P D, et al. Melatonin in plants [J]. Nutrition Reviews, 2001, 59(9): 286–290.
[29] Zhang N, Sun Q, Zhang H, et al. Roles of melatonin in abiotic stress resistance in plants [J]. Journal of Experimental Botany, 2015, 66(3): 647–656. DOI: 10.1093/jxb/eru336
[30] Park W J. Melatonin as an endogenous plant regulatory signal: debates and perspectives [J]. Journal of Plant Biology, 2011, 54(3): 143–149. DOI: 10.1007/s12374-011-9159-6
[31] Shi H, Chen Y, Tan D, et al. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis [J]. Journal of Pineal Research, 2015, 59(1): 102–108. DOI: 10.1111/jpi.12244
[32] Gong B, Li X, Bloszies S, et al. Sodic alkaline stress mitigation by interaction of nitric oxide and polyamines involves antioxidants and physiological strategies in Solanuml ycopersicum [J]. Free Radical Biology and Medicine, 2014, 71(6): 36–48.
[33] Arnao M B, Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? [J]. Trends in Plant Science, 2014, 19(12): 789–797. DOI: 10.1016/j.tplants.2014.07.006
[34] Hardeland R. Melatonin in plants-diversity of levels and multiplicity of functions [J]. Frontiers in Plant Science, 2016, 7(815769): 198–219.
[35] Nawaz M A, Huang Y, Bie Z, et al. Melatonin: current status and future perspectives in plant science [J]. Frontiers in Plant Science, 2015, 6(1230): 1230–1242.
[36] Zhang H, Zhang N, Yang R, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumissativus L.) [J]. Journal of Pineal Research, 2014, 57(3): 269–279. DOI: 10.1111/jpi.2014.57.issue-3
[37] Wang P, Sun X, Chang C, et al. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation [J]. Journal of Pineal Research, 2013, 55: 424–434.
[38] Byeon Y, Back K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield [J]. Journal of Pineal Research, 2014, 56(4): 408–414. DOI: 10.1111/jpi.2014.56.issue-4
[39] Park S, Back K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination [J]. Journal of Pineal Research, 2012, 53(4): 385–389. DOI: 10.1111/jpi.2012.53.issue-4
[40] Wang P, Sun X, Li C, et al. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple [J]. Journal of Pineal Research, 2013, 54(3): 292–302. DOI: 10.1111/jpi.2013.54.issue-3
[41] Marschner H. Mineral nutrition of higher plants (2nd edition)[M]. London: Academic Press, 1995.
[42] Li S W, Xue L, Xu S, et al. Mediators, genes and signaling in adventitious rooting [J]. Botanical Review, 2009, 75(2): 230–247. DOI: 10.1007/s12229-009-9029-9
[43] Negi S, Sukumar P, Liu X, et al. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato [J]. Plant Journal, 2010, 61(1): 3–15. DOI: 10.1111/tpj.2009.61.issue-1
[44] Malamy J E. Lateral root formation[A]. Beeckman T. Annual Plant Reviews, Volume 37: Root development[M]. Wiley–Blackwell, 2010. 83–126.
[45] Tyburski J, Tretyn A. The role of light and polar auxin transport in root regeneration from hypocotyls of tomato seedling cuttings [J]. Plant Growth Regulation, 2004, 42(1): 39–48. DOI: 10.1023/B:GROW.0000014896.18601.38
[46] Zhang N, Hasenstein K H. Initiation and elongation of lateral roots in Lactucasativa. [J]. International Journal of Plant Sciences, 160(3): 511–519.
[47] Werner T, Motyka V, Strnad M, et al. Regulation of plant growth by cytokinin [J]. Proceedings of the National Academy of Sciences of United States of America, 2001, 98(18): 10487–10492. DOI: 10.1073/pnas.171304098
[48] Hernández-Ruiz J, Cano A, Arnao M B. Melatonin acts as a growth-stimulating compound in some monocot species [J]. Journal of Pineal Research, 2005, 39(2): 137–142. DOI: 10.1111/j.1600-079X.2005.00226.x
[49] Chen Q, Qi W B R J, Wei W, et al. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea [J]. Journal of Plant Physiology, 2009, 166(3): 324–328. DOI: 10.1016/j.jplph.2008.06.002
[50] Sarropoulou V N, Therios I N. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunuscerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb) [J]. Journal of Pineal Research, 2012, 52(1): 38–46. DOI: 10.1111/jpi.2011.52.issue-1
[51] 李春俭. 高级植物营养学[M]. 北京: 中国农业大学出版社, 2015. Li C J. Advanced plant nutrition [M]. Beijing: China Agricultural University Press, 2015.
[52] Wang Q, An B, Wei Y, et al. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis [J]. Frontiers in Plant Science, 2016, 7(2587): 1882–1893.
[53] Shen J, Li C, Mi G, et al. Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China [J]. Journal of Experimental Botany, 2013, 64(5): 1181–1192. DOI: 10.1093/jxb/ers342
[54] Zhang F, Shen J, Zhang J, et al. Chapter one-rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China [J]. Advances in Agronomy, 2010, 107(6): 1–32.
[55] Shen J, Yuan L, Zhang J, et al. Phosphorus dynamics: from soil to plant [J]. Plant Physiology, 2011, 156(3): 997–1005. DOI: 10.1104/pp.111.175232
[56] Arnao M B, Hernández-Ruiz J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinusalbus L. [J]. Journal of Pineal Researd, 2007, 42(2): 147–152. DOI: 10.1111/jpi.2007.42.issue-2
[57] Koyama F C, Carvalho T L G, Alves E, et al. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum [J]. Journal of Eukaryotic Microbiology, 2013, 60(6): 646–651. DOI: 10.1111/jeu.12080
[58] Zuo B, Zheng X, He P, et al. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thalianaplants [J]. Journal of Pineal Research, 2014, 57(4): 408–417. DOI: 10.1111/jpi.2014.57.issue-4
[59] Zhang N, Zhao B, Zhang H J, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumissativus L.) [J]. Journal of Pineal Research, 2012, 54(1): 15–23.
[60] Wen D, Gong B, Sun S, et al. Promoting roles of melatonin in adventitious root development of Solanumlycopersicum L. by regulating auxin and nitric oxide signaling [J]. Frontiers in Plant Science, 2016, 7(925): 718–728.
[61] Sarropoulou V K D. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunusavium × Prunuscerasus) [J]. Plant Physiology and Biochemistry, 2012, 61: 162–168. DOI: 10.1016/j.plaphy.2012.10.001
[62] Sarrou E, Dimassi-Theriou K, Therios I. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punicagranatum cv. “Wonderful” [J]. Turkish Journal of Botany, 2014, 38: 293–301.
[63] Morris E C, Griffiths M, Golebiowska A, et al. Shaping 3D root system architecture [J]. Current Biology, 2017, 27(17): R919–R930. DOI: 10.1016/j.cub.2017.06.043
[64] Amtmann A, Shahzad Z. To respond or not to respond? Natural variation of root architectural responses to nutrient signals [J]. Journal of Experimental Botany, 2017, 68(11): 2636–2640. DOI: 10.1093/jxb/erx160
[65] Chen Z, Gu Q, Yu X, et al. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation [J]. Annual of Botany, 2018, 121(6): 1127–1136. DOI: 10.1093/aob/mcx207
[66] Liang C, Li A, Yu H, et al. Melatonin regulates root architecture by modulating auxin response in rice [J]. Frontiers in Plant Science, 2017, 8: 134–145.
[67] Arnao M B, Hernández-Ruiz J. Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin [J]. Acta Physiologiae Plantarum, 2017, 39(6): 127–136. DOI: 10.1007/s11738-017-2428-3
[68] Zhang R, Sun Y, Liu Z, et al. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress [J]. Journal of Pineal Research, 2017, 62(4): e12403–e12417. DOI: 10.1111/jpi.2017.62.issue-4
[69] Li C, Liang B, Chang C, et al. Exogenous melatonin improved potassium content in Malus under different stress conditions [J]. Journal of Pineal Research, 2016, 61(2): 218–229. DOI: 10.1111/jpi.12342
[70] Takahashi F, Sato-Nara K, Kobayashi K, et al. Sugar-induced adventitious roots in Arabidopsis seedlings [J]. Journal of Plant Research, 2003, 116(2): 83–91.
[71] 李淑钰, 李传友. 植物根系可塑性发育的研究进展与展望 [J]. 中国基础科学, 2016, 18(2): 14–21. DOI: 10.3969/j.issn.1009-2412.2016.02.002 Li S Y, Li C Y. Developmental plasticity of plant roots [J]. China Basic Science, 2016, 18(2): 14–21. DOI: 10.3969/j.issn.1009-2412.2016.02.002
[72] Noordwijk M V, Lusiana B. WaNuLCAS, a model of water, nutrient and light capture in agroforestry systems [J]. Agroforestry Systems, 1998, 43(1-3): 217–242.
[73] Ryan M G, Lavigne M B, Gower S T. Annual carbon cost of autotrophic respiration in boreal forest ecosystems in relation to species and climate [J]. Journal of Geophysical Research Atmospheres, 1997, 102(D24): 28871–28883. DOI: 10.1029/97JD01236
[74] Raich J W, Nadelhoffer K J. Belowground carbon allocation in forest ecosystems: global trends [J]. Global Change Biology, 2007, 13(10): 2089–2109. DOI: 10.1111/gcb.2007.13.issue-10
[75] Trumbore S E, Gaudinski J B. The secret lives of roots [J]. Science, 2003, 302(5649): 1344–1345. DOI: 10.1126/science.1091841
[76] Wolabu T W, Tadege M. Photoperiod response and floral transition in sorghum [J]. Plant Signaling and Behavior, 2016, 11(12): e1261232. DOI: 10.1080/15592324.2016.1261232
[77] Brambilla V, Gomezariza J, Cerise M, et al. The importance of being on time: regulatory networks controlling photoperiodic flowering in cereals [J]. Frontiers in Plant Science, 2017, 8: 665–673. DOI: 10.3389/fpls.2017.00665
[78] Tal O, Haim A, Harel O, et al. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. [J]. Journal of Experimental Botany, 2011, 62(6): 1903–1910. DOI: 10.1093/jxb/erq378
[79] Beilby M J, Turi C E, Baker T C, et al. Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in Characeae (Chara australis Brown) [J]. Plant Signal and Behavior, 2015, 10(11): e1261232-1.
[80] Shi H, Wei Y, He C. Melatonin-induced CBF/DREB1s, are essential for diurnal change of disease resistance and CCA1, expression in Arabidopsis [J]. Plant Physiology and Biochemistry, 2016, 100: 150–155. DOI: 10.1016/j.plaphy.2016.01.018
[81] Kolář J, Macháčková I, Eder J, et al. Melatonin: occurrence and daily rhythm in Chenopodium rubrum [J]. Phytochemistry, 1997, 44(8): 1407–1413. DOI: 10.1016/S0031-9422(96)00568-7
[82] Mahmud K P, Holzapfel B P, Guisard Y, et al. Circadian regulation of grapevine root and shoot growth and their modulation by photoperiod and temperature [J]. Journal of Plant Physiology, 2018, 222: 86–93. DOI: 10.1016/j.jplph.2018.01.006
[83] Weeda S, Zhang N, Zhao X, et al. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems [J]. PLoS ONE, 2014, 9(3): e93462. DOI: 10.1371/journal.pone.0093462
[84] Han Q H, Huang B, Ding C B, et al. Effects of melatonin on anti-oxidative systems and photosystem Ⅱ in cold-stressed rice seedlings [J]. Frontiers in Plant Science, 2017, 8: 785–798. DOI: 10.3389/fpls.2017.00785
[85] Dušan L, Murch S J, Beilby M J, et al. Exogenous melatonin affects photosynthesis in characeae Charaaustralis [J]. Plant Signaling and Behavior, 2013, 8(3): e23279-1–e23279-5. DOI: 10.4161/psb.23279
[86] Gibson S I. Control of plant development and gene expression by sugar signaling [J]. Current Opinion in Plant Biology, 2005, 8(1): 93–102. DOI: 10.1016/j.pbi.2004.11.003
[87] Lastdrager J, Hanson J, Smeekens S. Sugar signals and the control of plant growth and development [J]. Journal of Experimental Botany, 2014, 65(3): 799–807. DOI: 10.1093/jxb/ert474
[88] Weschke W, Panitz R, Gubatz S, et al. The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development [J]. Plant Journal for Cell and Molecular Biology, 2003, 33(2): 395–411. DOI: 10.1046/j.1365-313X.2003.01633.x
[89] Dekkers B J, Schuurmans J A, Smeekens S C. Glucose delays seed germination in Arabidopsis thaliana. [J]. Planta, 2004, 218(4): 579–588. DOI: 10.1007/s00425-003-1154-9
[90] Pego J V, Weisbeek P J, Smeekens S C. Mannose inhibits Arabidopsis germination via a hexokinase-mediated step [J]. Plant Physiology, 1999, 119(3): 1017–1023. DOI: 10.1104/pp.119.3.1017
[91] Hanson J, Johannesson H, Engström P. Sugar-dependent alterations in cotyledon and leaf development in transgenic plants expressing the HDZhdip gene ATHB13 [J]. Plant Molecular Biology, 2001, 45(3): 247–262. DOI: 10.1023/A:1006464907710
[92] Gibson S I. Sugar and phytohormone response pathways: navigating a signaling network [J]. Journal of Experimental Botany,2004, 55(395): 253–264.
[93] Liu J, Samac D A, Bucciarelli B, et al. Signaling of phosphorus deficiency induced gene expression in white lupin requires sugar and phloem transport [J]. Plant Journal, 2005, 41(2): 257–268.
[94] Zhao H, Su T, Huo L, et al. Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case [J]. Journal of Pineal Research, 2015, 59(2): 255–266. DOI: 10.1111/jpi.12258
[95] Burkle L, Hibberd J M, Quick W P, et al. The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves [J]. Plant Physiology, 1998, 118(1): 59–68. DOI: 10.1104/pp.118.1.59
[96] Gottwald J R, Krysan P J, Young J C, et al. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters [J]. Proceedings of the National Academy of Sciences of United States of America, 2000, 97(25): 13979–13984. DOI: 10.1073/pnas.250473797
[97] Taiz L, Zeiger E. Plant physiology[M]. Sunderland, UK: Sinauer Associates, 1998.
[98] Arnao M B, Hernández-Ruiz J. The physiological function of melatonin in plants [J]. Plant Signaling and Behavior, 2006, 1(3): 89–95. DOI: 10.4161/psb.1.3.2640
[99] Taiz L, Zeiger E. Plant physiology [J]. Quarterly Review of Biology, 2006, 167(4): 161–168.
[100] Casimiro I, Marchant A, Bhalerao R P, et al. Auxin transport promotes Arabidopsis lateral root initiation [J]. Plant Cell, 2001, 13(4): 843–852. DOI: 10.1105/tpc.13.4.843
[101] Woodward A W, Bartel B. Auxin: regulation, action, and interaction [J]. Annals of Botany, 2005, 95(5): 707–735. DOI: 10.1093/aob/mci083
[102] Falasca G, Zaghi D, Possenti M, et al. Adventitious root formation in Arabidopsis thaliana thin cell layers [J]. Plant Cell Reports, 2004, 23(1–2): 17–25.
[103] Cary A J, Liu W, Howell S H. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings [J]. Plant Physiology, 1995, 107(4): 1075–1082. DOI: 10.1104/pp.107.4.1075
[104] Růzicka K, Ljung K, Vanneste S, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution [J]. Plant Cell, 2007, 19(7): 2197–2212. DOI: 10.1105/tpc.107.052126
[105] Arnao M B, Hernández-Ruiz J. Inhibition of ACC oxidase activity by melatonin and indole-3-acetic acid in etiolated lupinhypocotyls[A]. Ramina A, Chang C, Giovannoni J, et al. Advances in plant ethylene research[M]. Springer Netherlands, 2007. 101–103.
[106] Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato [J]. Planta, 2004, 218(6): 900–905. DOI: 10.1007/s00425-003-1172-7
[107] Lombardo M C, Graziano M, Polacco J C, et al. Nitric oxide functions as a positive regulator of root hair development [J]. Plant Signaling Behavior, 2006, 1(1): 28–33. DOI: 10.4161/psb.1.1.2398
[108] Janiak A, Kwaśniewski M, Szarejko I. Gene expression regulation in roots under drought [J]. Journal of Experimental Botany, 2016, 67(4): 1003–1014. DOI: 10.1093/jxb/erv512
[109] Kudoyarova G R, Dodd I C, Veselov D S, et al. Common and specific responses to availability of mineral nutrients and water [J]. Journal of Experimental Botany, 2015, 66(8): 2133–2144. DOI: 10.1093/jxb/erv017
[110] Gregory P J. Plant roots: Growth, activity and interaction with soils[M]. Oxford: Blackwell Publishing Ltd, 2014.
[111] Bardgett R D, Mommer L, Vries F T D. Going underground: root traits as drivers of ecosystem processes [J]. Trends in Ecology and Evolution, 2014, 29(12): 692–699. DOI: 10.1016/j.tree.2014.10.006
[112] McNickle G G, Cahill J F. Plant root growth and the marginal value theorem [J]. Proceedings of the National Academy of Sciences of united states of Amarica, 2009, 106(12): 4747–4751. DOI: 10.1073/pnas.0807971106
[113] Venturi V, Keel C. Signaling in the rhizosphere [J]. Trends in Plant Science, 2016, 21(3): 187–198. DOI: 10.1016/j.tplants.2016.01.005
[114] Zamioudis C, Pieterse C M. Modulation of host immunity by beneficial microbes [J]. Molecular Plant-Microbe Interactions, 2012, 25(2): 139–150. DOI: 10.1094/MPMI-06-11-0179
[115] Boller T, He S Y. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens [J]. Science, 2009, 324(5928): 742–744. DOI: 10.1126/science.1171647
[116] Millet Y A, Danna C H, Clay N K, et al. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns [J]. Plant Cell, 2010, 22(3): 973–990. DOI: 10.1105/tpc.109.069658
[117] Pieterse C M J, Zamioudis C, Berendsen R L, et al. Induced systemic resistance by beneficial microbes [J]. Annual Review of Phytopathology, 2014, 52: 347–375.
[118] Pieterse C M J, Leon-Reyes A, Van der Ent S, et al. Networking by small-molecule hormones in plant immunity [J]. Nature Chemical Biology, 2009, 5: 308–316. DOI: 10.1038/nchembio.164
[119] Vos I A, Pieterse C M J, Van Wees S C M. Costs and benefits of hormone-regulated plant defences[C]. Lunar and Planetary Science Conference, 2013, 43–55.
[120] Sanon A, Andrianjaka Z N, Prin Y, et al. Rhizosphere microbiota interferes with plant–plant interactions [J]. Plant and Soil, 2009, 321(1/2): 259–278.
[121] Jiao J, Ma Y, Chen S, et al. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts [J]. Frontiers in Plant Science, 2016, 7: 1387–1400.
[122] Ma Y, Jiao J, Fan X, et al. Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars [J]. Frontiers in Plant Science, 2016, 7: 2068–2083.
-
期刊类型引用(2)
1. 蔡娜,葛莹,阮仁宗,李勇,庄翠珍. 基于经验正交变换的山地柑橘叶片光谱特征分析. 甘肃科学学报. 2023(02): 39-47 .  百度学术
百度学术
2. 高飞,王晓丽,胡林,樊景超,刘婷婷,闫燊,曹姗姗. 2016年辽宁兴城富士、华红、嘎啦苹果叶片光谱与图像数据集. 农业大数据学报. 2022(01): 109-113 .  百度学术
百度学术
其他类型引用(6)



 下载:
下载: