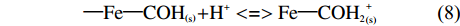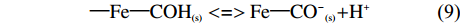Adsorption effectiveness of ammonium nitrogen by iron-modified rice husk biochars
-
摘要:目的
研究稻壳生物炭和3种铁改性稻壳生物炭对铵态氮的吸附特性,为其作为添加剂进行炭基肥料的开发提供参考。
方法以稻壳为原料,在500℃无氧条件下热解制备稻壳生物炭(RBC),并采用3种工艺制备铁改性稻壳生物炭 (FDRBC、FWRBC和FWBC)。利用比表面积测定仪 (BET) 和扫描电镜 (SEM)、X射线衍射 (XRD)、傅立叶红外光谱 (FT-IR) 等技术对稻壳炭和3种铁改性稻壳炭进行物理性质表征。以稻壳生物炭和3种铁改性稻壳生物炭为材料进行铵态氮吸附试验,采用Langmuir和Freundlich方程对稻壳炭和3种铁改性稻壳炭的等温吸附数据进行拟合;并分别用准一级动力学模型和准二级动力学模型对吸附数据进行拟合。
结果1) 经过铁改性,稻壳炭比表面积降低了2.4%~63.7%,孔径平均提高了2.8%~319.2%,pH均降低到5左右;2) FWBC和FWRBC在pH为6时,对NH4+-N的吸附量最大,FDRBC和RBC在pH为7时,对NH4+-N的吸附量最大;3) Langmuir吸附等温方程能够很好地拟合稻壳炭和3种铁改性稻壳炭对铵态氮的吸附数据,RBC、FDRBC、FWRBC和FWBC对铵态氮的最大吸附量分别为2.22、8.82、4.67和3.67 mg/g;4) 稻壳炭和3种铁改性稻壳炭对铵态氮的吸附行为符合准二级动力学方程。
结论供试稻壳炭和3种铁改性稻壳炭对铵态氮的吸附主要为单分子层吸附,以化学吸附方式为主。铁改性处理提高了稻壳炭的孔径,降低了pH。对铵态氮的吸附能力以FDRBC最优,用其制备新型肥料可提高肥料的保肥供肥能力。
Abstract:ObjectivesThe research studied the adsorption characteristics of rice husk biochar and three iron-modified rice husk biochar to ammonium nitrogen. The aim was to provide a basis for using them as carbon additives to manufacture new fertilizers.
MethodsBiochars (RBC, and FDRBC, FWRBC, FWBC) were produced from rice husk without and with FeCl3 through pyrolysis under an oxygen-limited condition at 500℃. The physico-chemical properties of biochar were detected by a specific surface area analyzer (BET), scanning electron microscopy (SEM), X-ray diffractometer (XRD), and Fourier transforms infrared spectroscopy (FT-IR). A chamber adsorption test was carried out using the rice husk biochar and three iron-modified rice husk biochars. Langmuir and Freundlich’s equations were used to fit the isotherm adsorption data of rice husk charcoal and three kinds of iron-modified rice husk charcoal. The quasi-first-order kinetic model and quasi-second-order kinetic model were used to fit the adsorption data, respectively.
Results1) The iron-modified rice husk charcoal’s specific surface area was reduced by 2.4%–63.7%, the average pore diameter increased by 2.8%–319.2%, and the pH reduced to about 5. 2) The adsorption ability of NH4+-N by FWRBC and FWBC was the best under the experimental condition with a pH of 6, while FDRBC and RBC had the best adsorption effect on NH4+-N when the pH was 7. Moreover, the Langmuir equation could better simulate the process of isothermal adsorption of NH4+-N by the 4 kinds of biochar, indicating that the adsorption of NH4+-N by the biochars was mainly due to monomolecular adsorption. 3) The maximum adsorption capacity of RBC, FDRBC, FWRBC and FWBC for ammonium nitrogen were 2.22, 8.82, 4.67, and 3.67 mg/g, respectively. 4) The pseudo-second-order kinetics model better defined the kinetics of the 4 kinds of biochar adsorption of ammonium nitrogen.
ConclusionsThe adsorption of rice husk charcoal and the three iron-modified rice husk charcoals on ammonium nitrogen is mainly monomolecular layer adsorption, a subset of chemical adsorption. Iron-modification process enhances aperture and pH of rice husk biochar to a different extent. FDRBC shows the best adsorption capacity of ammonium nitrogen, so it is most prospective in increasing fertilizer’s retention ability as an additive.
-
Keywords:
- rice husk biochar /
- iron-modified /
- ammonium nitrogen /
- adsorption
-
氮肥是植物所需求的最重要的肥料种类之一,对农作物的生长发育起着重要作用。近年来,有关氮素平衡施肥的研究也越来越多,但是在实际生产中氮肥利用效率并没有得到显著提高[1]。在氮肥的施用过程中出现了资源浪费、土壤退化、生态破坏以及环境恶化等一系列问题[2]。氮素对于植物来说是必不可少的,然而其相应的养分离子却极易被淋溶损失,这是氮肥利用率低的重要原因,也是导致水体富营养化,产生环境危害的重要因素[3]。如何有效地减少氮素流失到环境中是遏制环境恶化的关键,也是提高氮肥利用效率的有效途径。如今已经有了一些卓有成效的方法,如缓释肥料、包膜控释肥料的开发与应用,这些肥料的应用均起到了延缓养分释放,提高肥效以达到减少养分流失的作用[4]。
近年来,生物炭作为缓释肥料的载体已经被广泛应用,在农业和环境领域具有优良的前景。生物炭是由农作物秸秆或者其他生物质在无氧或限氧条件下热解而得,具有较大的比表面积和丰富的孔隙结构,同时在热解过程中,生物炭表面还会生成丰富的官能团结构,例如羧基、羟基等,这些特性使得生物炭具有良好的吸附性能[5]。生物炭可以钝化土壤重金属,增强土壤持水性能,减少养分流失[6-7]。有研究表明,生物炭对铵态氮具有很好的吸附效果,在土壤中添加0.5%的生物炭可以使土壤铵态氮的浸出率降低15%[8]。生物炭在水土两相中对铵态氮均有很好的吸附效果,但是原生生物炭的吸附能力还是较弱[9]。为了提高生物炭对铵态氮的吸附能力,人们采用了各种改性方法将阳离子负载到生物炭材料中,常用的改性方法有氧化改性、还原改性、酸碱改性以及金属负载改性等化学方法和高温热处理改性、微波改性等物理方法[10]。但是氧化改性、还原改性、酸碱改性以及微波改性等方式在规模化的实际生产中均存在生产成本高和工艺复杂的缺点。金属负载改性生物炭因其生产成本低、工艺简单以及优良的吸附特性,而具有很好的应用前景。金属铁负载改性生物炭也有多种制备工艺,主要包括干式混合热解法、湿式浸渍热解法和酸碱浸渍改性法,不同制备工艺的铁改性生物炭对铵态氮的吸附效果也各不相同[11-13]。王思源等[12]采用酸浸渍改性法制备的铁改性生物炭对铵态氮的吸附性能较未改性的生物炭提高了24.1%。智燕彩等[14]采用湿式浸渍热解法制备的铁改性花生生物炭对氮素的最大吸附量为41.58 mg/g,吸附性能有了显著提高。
以往的研究多集中在同一制备工艺下铁改性生物炭对铵态氮吸附性能上,但对不同制备工艺铁改性生物炭的理化特征与它们对铵态氮的吸附特性研究报道较少。以农业废弃物稻壳为原料制备生物炭材料,研究多种工艺制备的铁改性生物炭理化性质及其对铵态氮的吸附特性,为炭基肥料的开发与应用提供技术指导。
1. 材料与方法
1.1 供试材料
本试验所采用的农业废弃物稻壳来自浙江省海宁市杨渡科研基地,稻壳经去离子水洗涤去除杂质后烘干备用。试验所用药品包括硫酸铵 [(NH4)2SO4]、氯化铁 (FeCl3·6H2O)、盐酸 (HCl) 均为分析纯。
1.2 生物炭的制备
稻壳生物炭和3种铁改性稻壳生物炭的制备方法如下:1) 将稻壳放入管式炭化炉中,在500℃无氧条件下恒温热解90 min,冷却至室温后称重计算其产率,编号为RBC。2) 干式混合热解法,采用祝天宇等[11]的方法,称取稻壳和FeCl3·6H2O质量比为1∶1,将其研磨粉碎混合均匀放入管式炭化炉中,在500℃无氧条件下恒温热解90 min,冷却至室温后称重计算其产率,编号为FDRBC。3) 湿式浸渍热解法,采用智燕彩等[14]的方法,将稻壳研磨粉碎,用饱和FeCl3·6H2O溶液浸泡1 h,1 g稻壳对应FeCl3·6H2O溶液10 mL,然后用去离子水冲洗、烘干,将烘干后的稻壳放入管式炭化炉中,在500℃无氧条件下恒温热解90 min,冷却至室温后称重计算其产率,编号为FWRBC。4) 酸碱浸渍改性法,采用王思源等[12]的方法,将稻壳放入管式炭化炉中,在500℃无氧条件下恒温热解90 min,冷却至室温得到稻壳炭,然后将稻壳炭置于盐酸溶液中浸泡1 h,1 g稻壳炭对应浓度为1 mol/L的盐酸溶液10 mL,用去离子水淋洗、烘干。设置Fe3+与生物炭的质量比值为1.0,将浸泡烘干后的稻壳炭放置于FeCl3溶液中,调节溶液呈碱性,置于恒温震荡摇床中震荡1 h,用去离子水淋洗3次,最后75℃烘干,得到FeCl3改性稻壳炭,编号为FWBC。
1.3 氨储备液的配制
准确称取 (NH4)2SO4 (分析纯) 4.717 g于烧杯中,加入适量去离子水溶解,定容至1 L,获得1000 mg/L的NH4+储备液,室温保存备用。
1.4 生物炭理化性质表征
pH测定:取0.5 g生物炭于烧杯中,加入去离子水,土水比为1∶20,振荡10 min后静置0.5 h,使用pH计 (METTLER TOLEDO FE28) 测定。使用元素分析仪 (Vario ISOTOPE CUBE) 测定生物炭的C、N、H含量;使用X射线能谱仪 (Horiba 7021- H) 测定生物炭的O含量。生物炭比表面积通过比表面积测定仪 (TriStar II 3020 Version 3.02) 测定。生物炭的表面形态和官能团结构通过扫描电镜 (HITACHIS-4800) 和傅里叶变换红外光谱仪 (Nicolet 380) 分析测定。生物炭的物像由X射线衍射仪 (Bruker D8 Advance) 进行分析。
1.5 改性稻壳炭对铵态氮的吸附试验
1.5.1 pH环境对生物炭吸附铵态氮的影响
将铵储备液稀释至50 mg/L,用HCI和NaOH调节pH至3、4、5、6、7、8、9。分别称取0.1 g 改性稻壳炭放入小白瓶中,加入50 mL pH不同的溶液,放于摇床室温150 r/min震荡3 h,用碳氮分析仪 (multi C/N 3100) 测定滤液中铵态氮浓度,计算稻壳炭和3种铁改性稻壳炭对铵态氮的吸附量,确定最适pH。
1.5.2 反应时间对生物炭吸附铵态氮的影响
稀释铵储备液至50 mg/L,调节到最适pH。分别称取0.1 g稻壳炭和3种铁改性稻壳炭于小白瓶中,加入50 mL准备好的储备液,放于摇床在室温150 r/min震荡不同时间 (10 min、0.5 h、1 h、2 h、3 h、6 h、9 h及12 h) 后过滤,用碳氮分析仪测定滤液中铵态氮浓度,计算稻壳炭和3种铁改性稻壳炭对铵态氮的吸附量。
1.5.3 初始浓度对生物炭吸附铵态氮的影响
将铵储备液稀释至10、20、50、100、150、200 mg/L,调节至最适pH。分别称取0.1 g稻壳炭和3种铁改性稻壳炭于小白瓶中,加入50 mL准备好的储备液,放于摇床在室温150 r/min震荡3 h后过滤,用碳氮分析仪测定滤液中铵态氮浓度,计算稻壳炭和3种铁改性稻壳炭对铵态氮的吸附量。
生物炭对铵态氮的吸附量采用式 (1) 计算:
qe=V/M(C0−Ce) (1) 式中,qe—平衡吸附量 (mg/g);C0—溶液中铵态氮的初始浓度 (mg/L);Ce—吸附后溶液中铵态氮的平衡浓度 (mg/L);V—铵态氮溶液的体积 (L);M为生物炭质量 (g)。
1.6 生物炭对NH4+的吸附等温线
分别用Langmuir、Freundlich模型对稻壳炭和3种铁改性生物炭的等温吸附数据进行拟合,Langmuir等温方程如 (2)、(3) 所示[15],
qe=qmax (2) 转换线性方程为:
\frac{{C_e}}{{q_e}} = \frac{1}{{q_{\max} K_L}} + \frac{{C_e}}{{q_{\max} }} (3) 式中,qe—平衡吸附量 (mg/g);Ce—平衡溶液浓度 (mg/L);qmax—最大吸附量 (mg/g);KL—Langmuir吸附常数。
qmax
和KL可由方程中的斜率和截距求得。 Freundlich等温方程如 (4)、(5) 所示
q_e = K_FC_e{^{1/n}} (4) 转换为线性方程为:
\ln q_e = \ln K_F + \frac{1}{n}\ln C_e (5) 式中,qe—平衡吸附量 (mg/g);Ce—平衡溶液浓度 (mg/L);KF—Freundlich吸附常数;n—组分因子,无量纲。
KF和n可根据方程的斜率和截距求得。
1.7 生物炭对NH4+的吸附动力学
将吸附试验的数据分别用准一级动力学模型和准二级动力学模型进行拟合,分析NH4+的吸附动力学行为。准一级动力学模型方程和准二级动力学模型方程如式 (6)、(7) 所示[16]。
\lg (q_e - q_t) = \lg q_e - \frac{{tk_1}}{{2.303}} (6) \frac{t}{{q_t}} = \frac{1}{{k_2{q_e^2}}} + \frac{t}{{q_e}} (7) 式中:qe—平衡吸附量 (mg/g);qt—t时的吸附量 (mg/g);k1—准一级吸附速率常数 (min–1);k2—准二级吸附速率常数[g/(mg·min)]。
1.8 数据处理与分析
试验数据使用Excel 2010 进行处理,用SPSS 19进行单因素方差分析,使用Origin 85软件作图。
2. 结果与分析
2.1 生物炭的表征
2.1.1 生物炭的基本理化性质
由稻壳炭和3种改性生物炭的基本理化性质(表1)可知,铁改性稻壳生物炭FDRBC、FWRBC和FWBC相对于稻壳炭来说BET比表面积分别降低了43.5%、2.4%和63.7%,孔径平均提高了42.8%~319.2%,其中FDRBC的孔径较稻壳炭提高了319.2%。铁改性稻壳炭的BET比表面积降低、孔径增加,表明在改性过程中,生物炭内部大孔得以形成,小孔遭到破坏,中孔和大孔得到疏通。3种铁改性稻壳炭FDRBC、FWRBC和FWBC的pH较稻壳炭显著降低,均在5左右。铁改性后生物炭氮(N)含量无显著变化,碳(C)和氢(H)含量差异显著。H/C是表征吸附剂芳香性大小的重要指标,其值越小芳香性越高;O/C是表征吸附剂亲水性大小的重要指标,其值越大亲水性越大。3种铁改性炭的H/C和O/C均显著高于未改性的稻壳炭。
表 1 生物炭的基本理化性质Table 1. Basic physical and chemical properties of biochar样品
SampleBET比表面积 (m2/g)
BET specific surface area孔径 (nm)
Average pore sizepH RBC 12.4 ± 1.2 a 8.72 ± 1.6 c 10.70 ± 0.6 a FDRBC 7.0 ± 0.7 b 36.55 ± 2.8 a 4.61 ± 0.4 b FWRBC 12.1 ± 0.9 a 8.96 ± 2.4 bc 5.08 ± 0.3 b FWBC 4.5 ± 0.6 c 12.11 ± 0.9 b 5.44 ± 0.3 b 样品
SampleN (%) C (%) H (%) O (%) H/C O/C RBC 0.72 ± 0.02 a 57.73 ± 1.5 a 3.82 ± 0.05 c 7.9 ± 0.6 a 0.066 ± 0.002 c 0.137 ± 0.001 b FDRBC 0.70 ± 0.03 a 40.63 ± 3.1 c 3.91 ± 0.14 c 6.8 ± 0.4 b 0.096 ± 0.007 a 0.167 ± 0.005 a FWRBC 0.71 ± 0.05 a 51.49 ± 0.9 b 4.14 ± 0.07 b 8.4 ± 0.5 a 0.080 ± 0.003 b 0.163 ± 0.002 a FWBC 0.72 ± 0.02 a 49.68 ± 2.3 b 4.61 ± 0.05 a 8.4 ± 0.7 a 0.093 ± 0.006 a 0.169 ± 0.007 a 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method; 同列数据后不同字母表示不同处理间差异显著 (P < 0.05) Values followed by different letters in a column indicate significant difference among treatments (P < 0.05). 2.1.2 表面形态结构
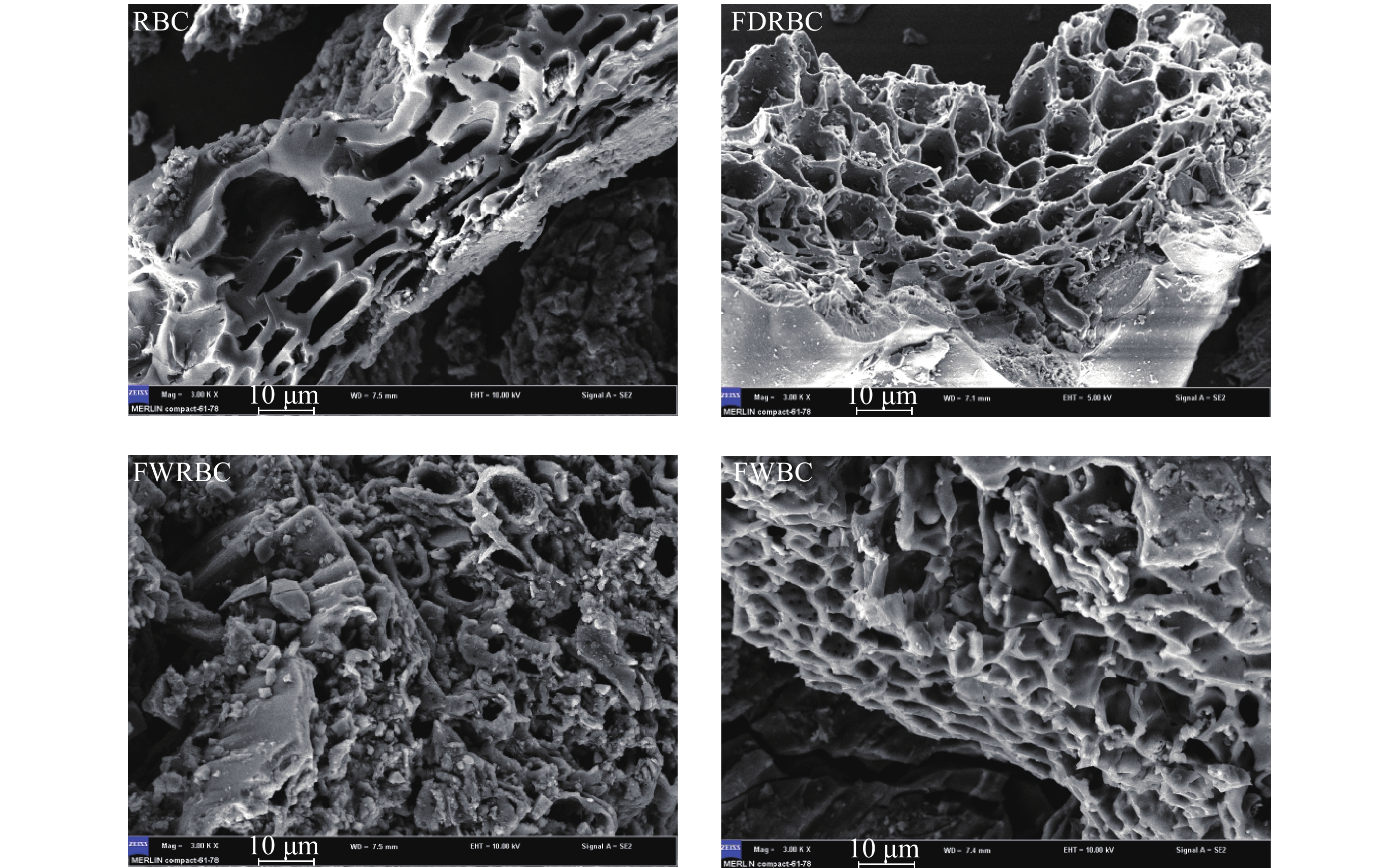
由稻壳炭和3种铁改性生物炭的扫描电镜图(图1)可以看出,铁改性对稻壳炭的表面结构有较大的影响。几种生物炭相比较而言,未改性稻壳炭空间排列较为整齐,孔隙结构表面光滑清晰可见。经铁改性之后的稻壳炭FDRBC、FWRBC和FWBC表面结构变得杂乱无章,孔隙结构变得无序不规则。孔隙表面负载了金属或金属氧化物,使得小孔隙堵塞。这也进一步印证了铁改性后稻壳炭BET比表面积减少,平均孔径增加的结果(表1)。
![]() 图 1 稻壳炭和3种铁改性稻壳炭样品扫描照片[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 1. SEM images of rice husk biochar and three types of iron-modified rice husk biochar
图 1 稻壳炭和3种铁改性稻壳炭样品扫描照片[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 1. SEM images of rice husk biochar and three types of iron-modified rice husk biochar2.1.3 X射线衍射分析
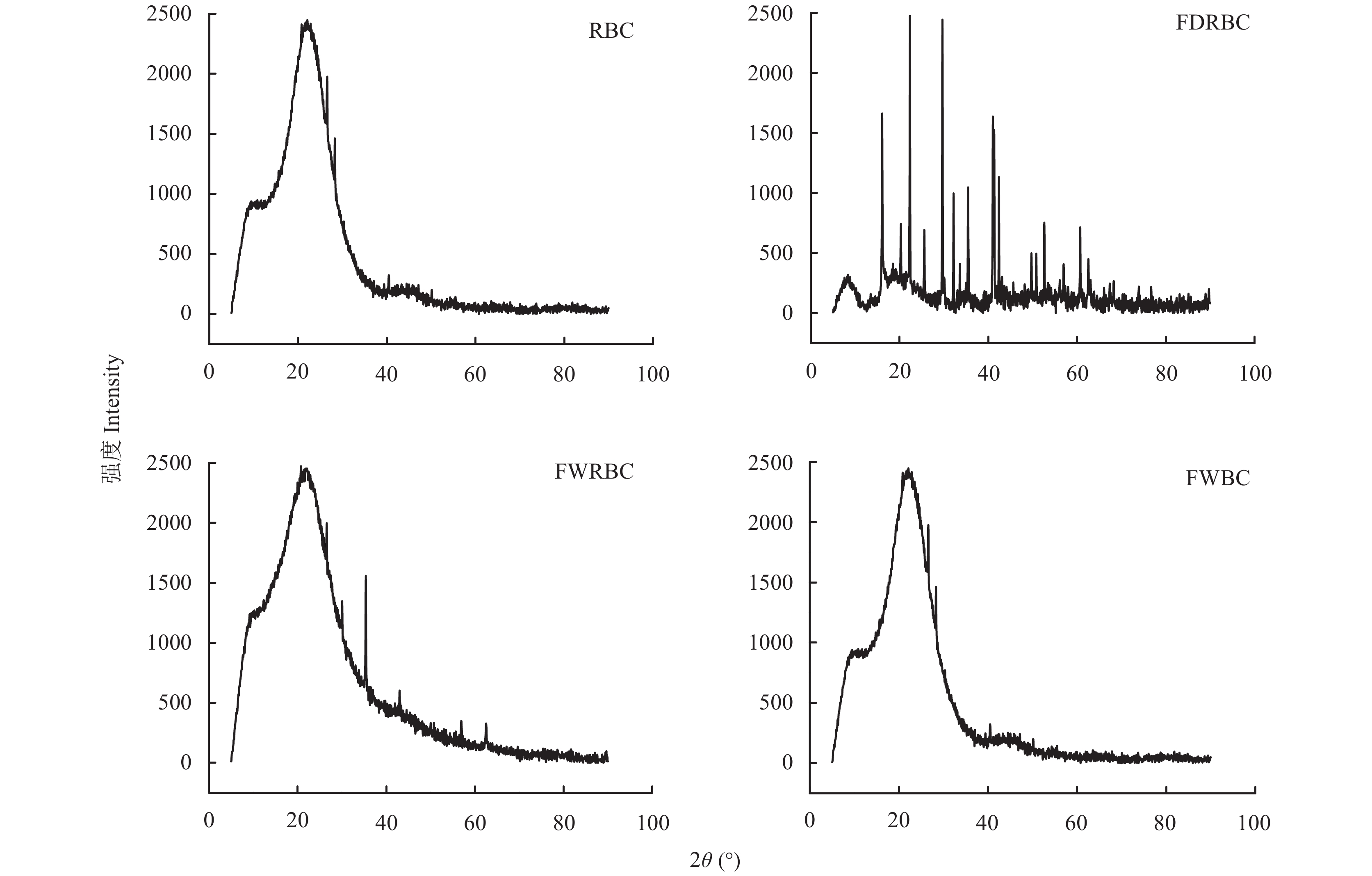
采用X衍射线技术 (XRD) 对稻壳炭和3种铁改性炭进行表征研究其晶体结构差异,其结果如图2所示。RBC、FWRBC和FWBC的XRD图谱在23.0°附近出现较宽的衍射峰,这是碳的特征峰,主要是由于纤维素的存在。RBC和FWBC图谱相似,说明FWBC与稻壳炭之间无显著差异,FWBC中负载的铁离子较少;并且图谱中其他的衍射峰较少,说明结晶结构较少。FDRBC存在较多的衍射峰,2θ=16.10°、20.32°、25.60°、29.68°、32.18°、33.62°、35.44°、41.04°、41.34°、42.40°、49.72°、50.82°、52.62°、60.70°、62.56°,说明为无取向多晶物质。其中2θ=35.44°、52.62°、60.70°和62.56°对应的物质为Fe3O4,特别是35.44°的峰表明存在γ-Fe2O3。FWRBC在2θ=35.38°处存在衍射峰,表明生物炭中负载了少量的Fe3O4。
![]() 图 2 稻壳炭和3种铁改性稻壳炭X射线衍射图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 2. XRD pattern of rice husk biochar and three types of iron-modified rice husk biochar
图 2 稻壳炭和3种铁改性稻壳炭X射线衍射图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 2. XRD pattern of rice husk biochar and three types of iron-modified rice husk biochar2.1.4 傅里叶红外光谱分析
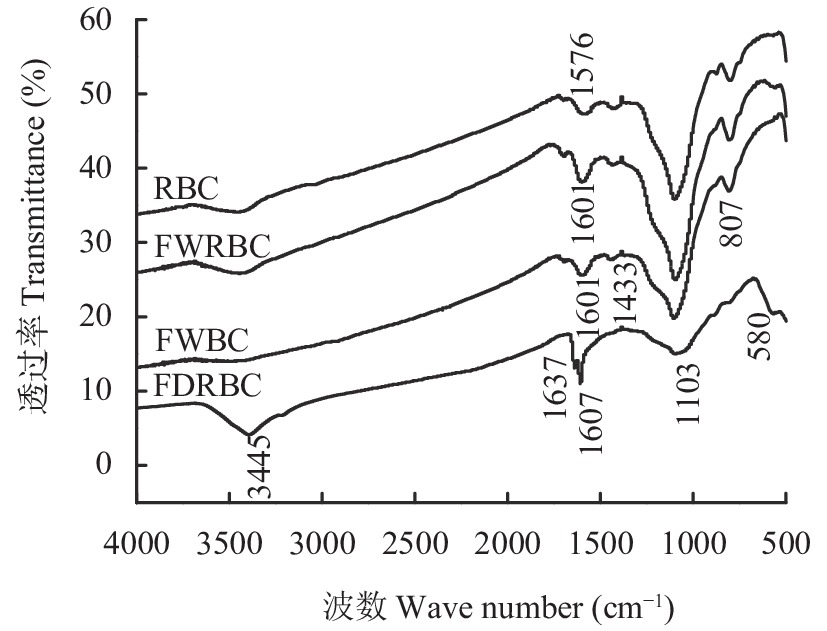
傅里叶红外光谱 (FT-IR) 是表征和鉴别生物质的重要方法。根据图3中RBC和FWRBC的红外光谱图谱对比可知,RBC和FWRBC的FT-IR光谱图趋势大体一致,表明它们含有相似的官能团结构。稻壳经过FeCl3溶液浸泡改性后,制备的生物炭表面官能团结构未发生显著变化。RBC、FDRBC和FWRBC在3445 cm–1附近存在吸收峰,表明了―OH官能团的存在,或者存在醛或醇类物质。FWBC在3445 cm–1不存在吸收峰,说明酸碱浸渍改性会降低生物炭―OH官能团的含量。FDRBC在1607.72 cm–1和1637.97 cm–1处存在两处吸收峰,表明FDRBC较其他3种生物炭含有种类更多的芳香基团。RBC、FWRBC和FWBC在1433 cm–1处均存在吸收峰,这是由C―H面内弯曲振动引起的,说明RBC、FWRBC和FWBC存在烷烃。4种生物炭在1100 cm–1附近均存在吸收峰,这是由C―O伸缩振动引起的,表明4种生物炭中含有醇类物质。RBC在801.47 cm–1处、FWRBC在807.21 cm–1处、FWBC在808.15 cm–1处出现较弱的吸收峰,这是由C―H面外弯曲振动引起的。FDRBC在580 cm–1处出现较弱的吸收峰,这是Fe―O振动的特征吸收峰,说明FDRBC中负载有Fe3O4,这也印证了2.1.3中FDRBC的X射线衍射图。
![]() 图 3 稻壳炭和3种铁改性稻壳炭FT-IR光谱图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 3. FT-IR spectra of rice husk biochar and three types of iron-modified rice husk biochar
图 3 稻壳炭和3种铁改性稻壳炭FT-IR光谱图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 3. FT-IR spectra of rice husk biochar and three types of iron-modified rice husk biochar2.1.5 氮气吸附/脱附及孔径分布
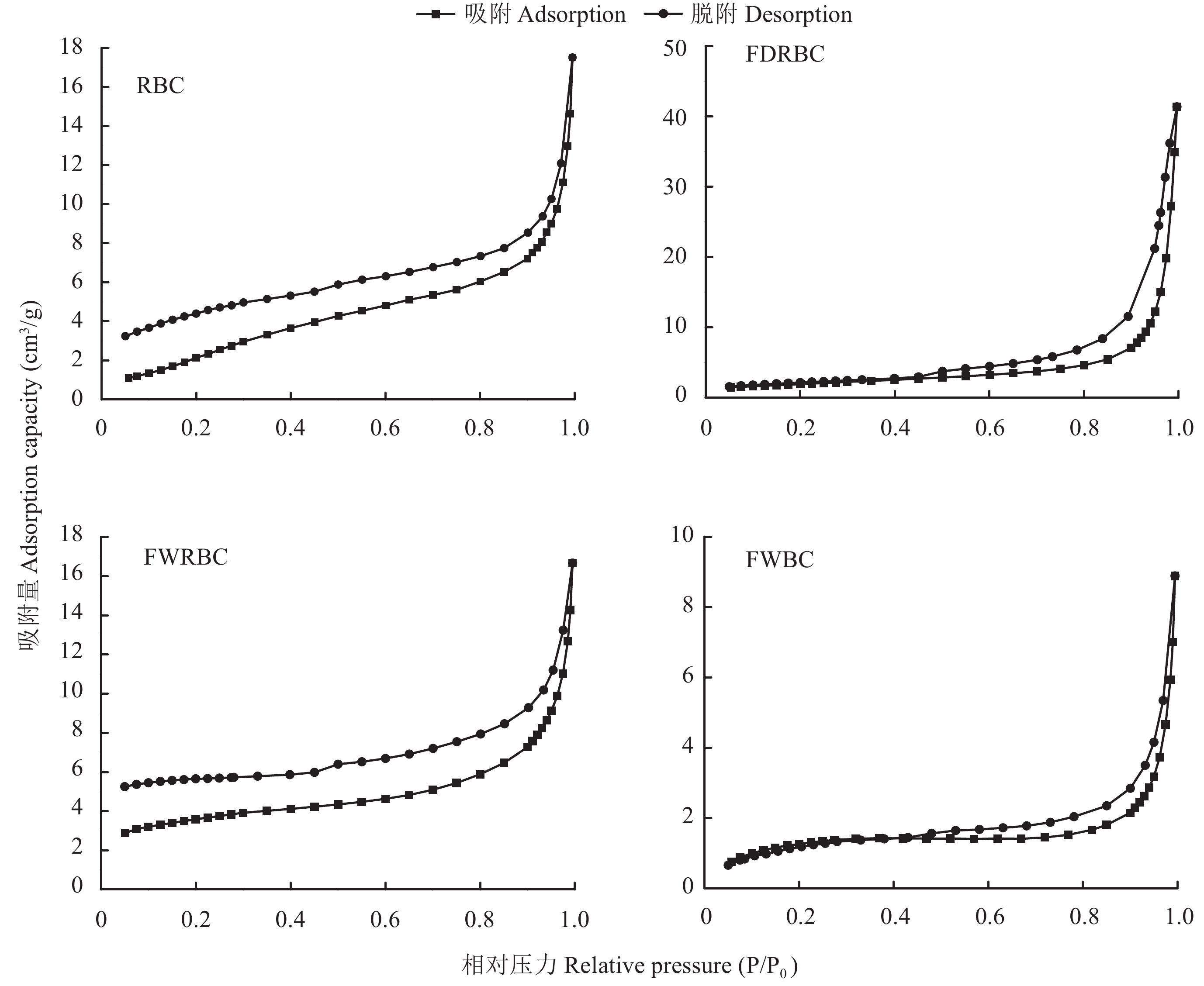
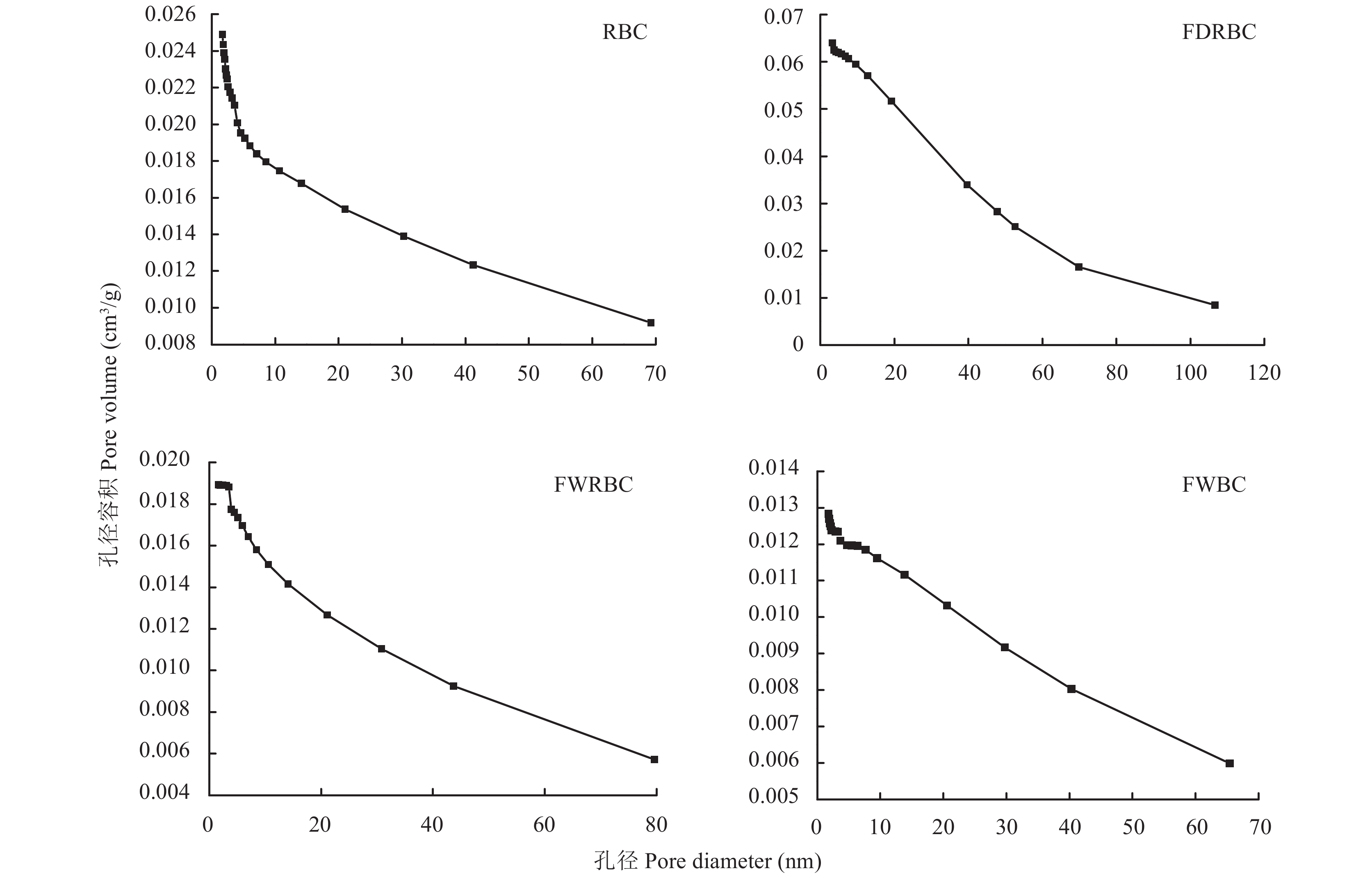
由图4可以看出,FDRBC和FWBC的吸附/脱附滞后循环均出现在0.5~1.0 P/P0范围,等温线属于IV型,且具有H3型回滞环,表明这两种改性稻壳炭样品具有介孔结构,且孔隙结构很不规整。RBC和FWRBC同样属于Ⅳ型等温线,但是不具有回滞环,表明RBC和FWRBC孔隙结构较为规整。RBC的平均孔径为8.72 nm,总孔容积为0.026 cm3 / g;FDRBC的平均孔径为36.55 nm,总孔容积为0.064 cm3 / g,其平均孔径和总孔容积较RBC分别提高了319.2%和146.2%。FWRBC与RBC差异不明显;FWBC的总孔容积为0.013 cm3 / g,较RBC下降了 50% (图5)。
![]() 图 4 稻壳炭和3种铁改性稻壳炭氮气吸附/脱附等温线[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 4. Nitrogen adsorption-desorption isotherms of rice husk biochar and three types of iron-modified rice husk biochar
图 4 稻壳炭和3种铁改性稻壳炭氮气吸附/脱附等温线[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 4. Nitrogen adsorption-desorption isotherms of rice husk biochar and three types of iron-modified rice husk biochar![]() 图 5 稻壳炭和3种铁改性稻壳炭孔径分布图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 5. Pore size distributions of rice husk biochar and three types of iron-modified rice husk biochar
图 5 稻壳炭和3种铁改性稻壳炭孔径分布图[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 5. Pore size distributions of rice husk biochar and three types of iron-modified rice husk biochar2.2 pH对生物炭吸附NH4+的影响
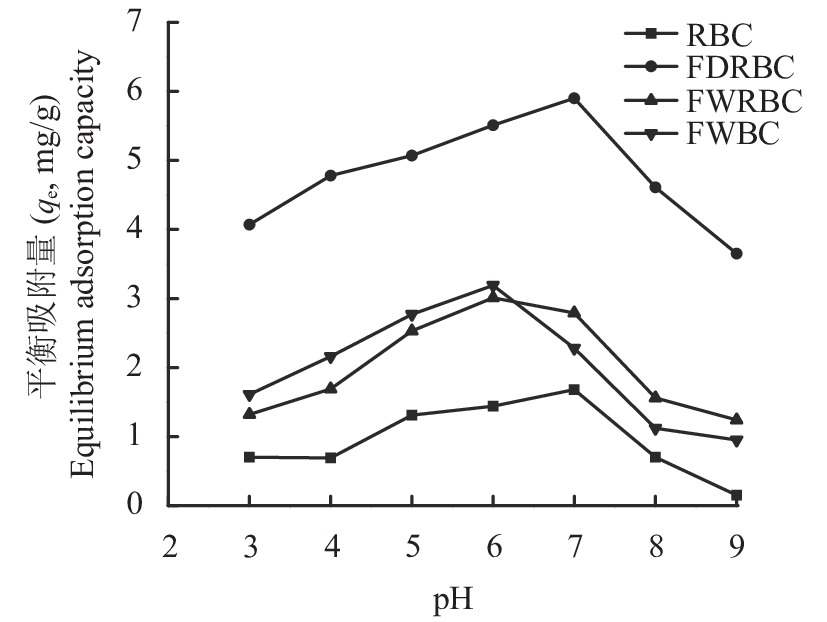
我国土壤类型复杂多样,不同地区土壤pH也不尽相同,酸碱各异,生物炭施入不同类型的土壤对铵态氮的吸附效果也有差异。因此了解生物炭在不同pH条件下对铵态氮的吸附效果,对于指导炭基肥料的施用具有重要的现实意义。由稻壳炭和3种铁改性生物炭在不同pH环境下对NH4+-N的平衡吸附量变化规律(图6)可知,随着pH的升高,稻壳炭和3种铁改性生物炭对NH4+-N的吸附量呈先升高后降低的趋势,当pH为6时,FWBC和FWRBC对NH4+-N的吸附效果最佳,其最大吸附量分别为3.19和3.01 mg/g;当pH为7时,FDRBC和RBC对NH4+-N的吸附效果最佳,其最大吸附量分别为5.90和1.68 mg/g。
![]() 图 6 pH 对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 6. Effects of pH on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 6 pH 对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 6. Effects of pH on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar2.3 反应时间对生物炭吸附NH4+的影响
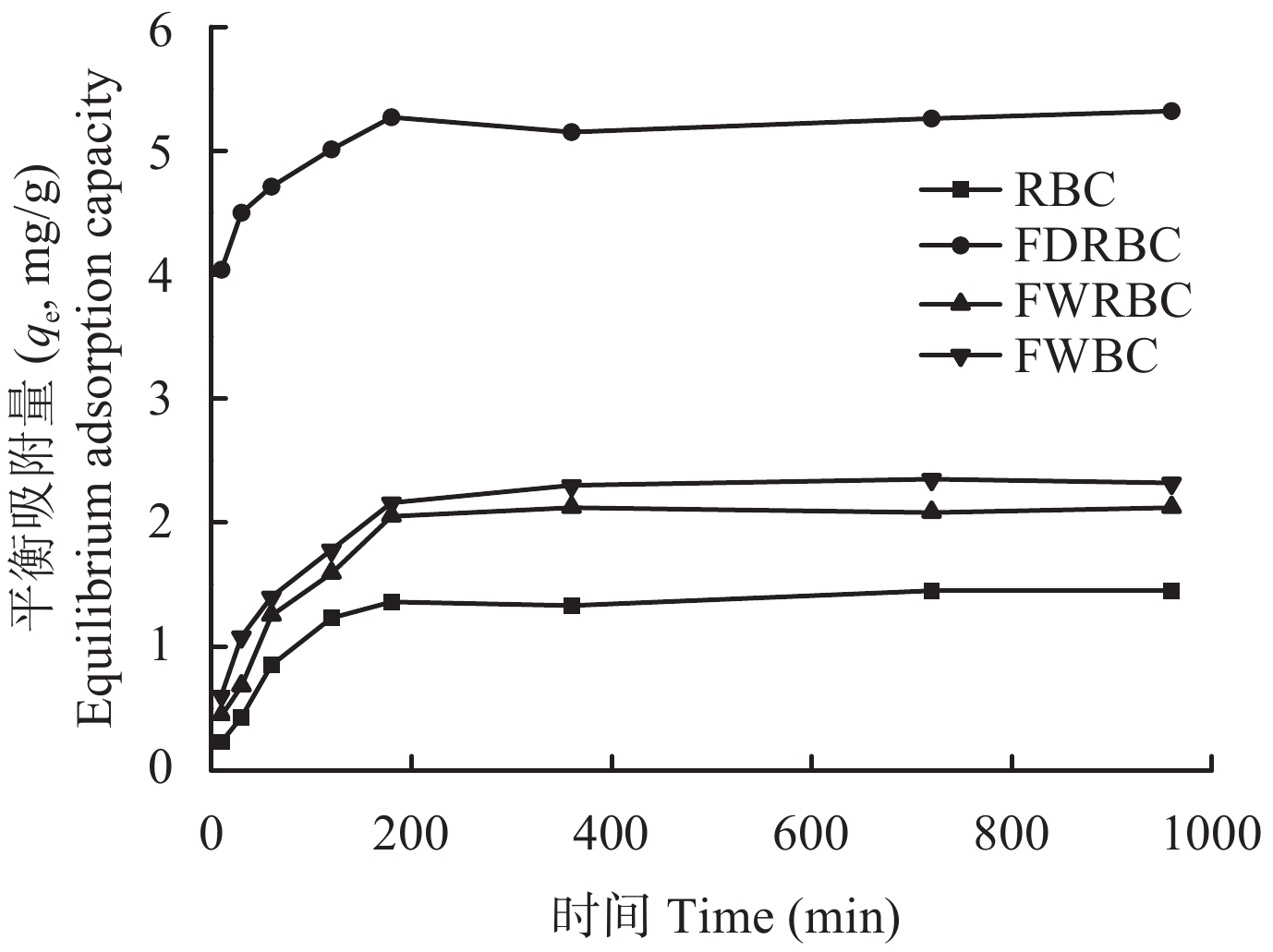
图7表明,3种铁改性生物炭对铵态氮的吸附变化趋势和稻壳炭大体一致,反应初期吸附量迅速增加之后趋于平衡。FDRBC、FWRBC和FWBC在吸附时间为180 min时,吸附反应达到动态平衡;稻壳炭在反应时间为120 min时,吸附反应达到动态平衡。稻壳炭和3种铁改性生物炭达到吸附平衡后对铵态氮的吸附量表现为FDRBC > FWBC > FWRBC > RBC。
![]() 图 7 反应时间对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 7. Effects of contact time on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 7 反应时间对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 7. Effects of contact time on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar2.4 铵态氮初始溶液浓度对生物炭吸附NH4+的影响
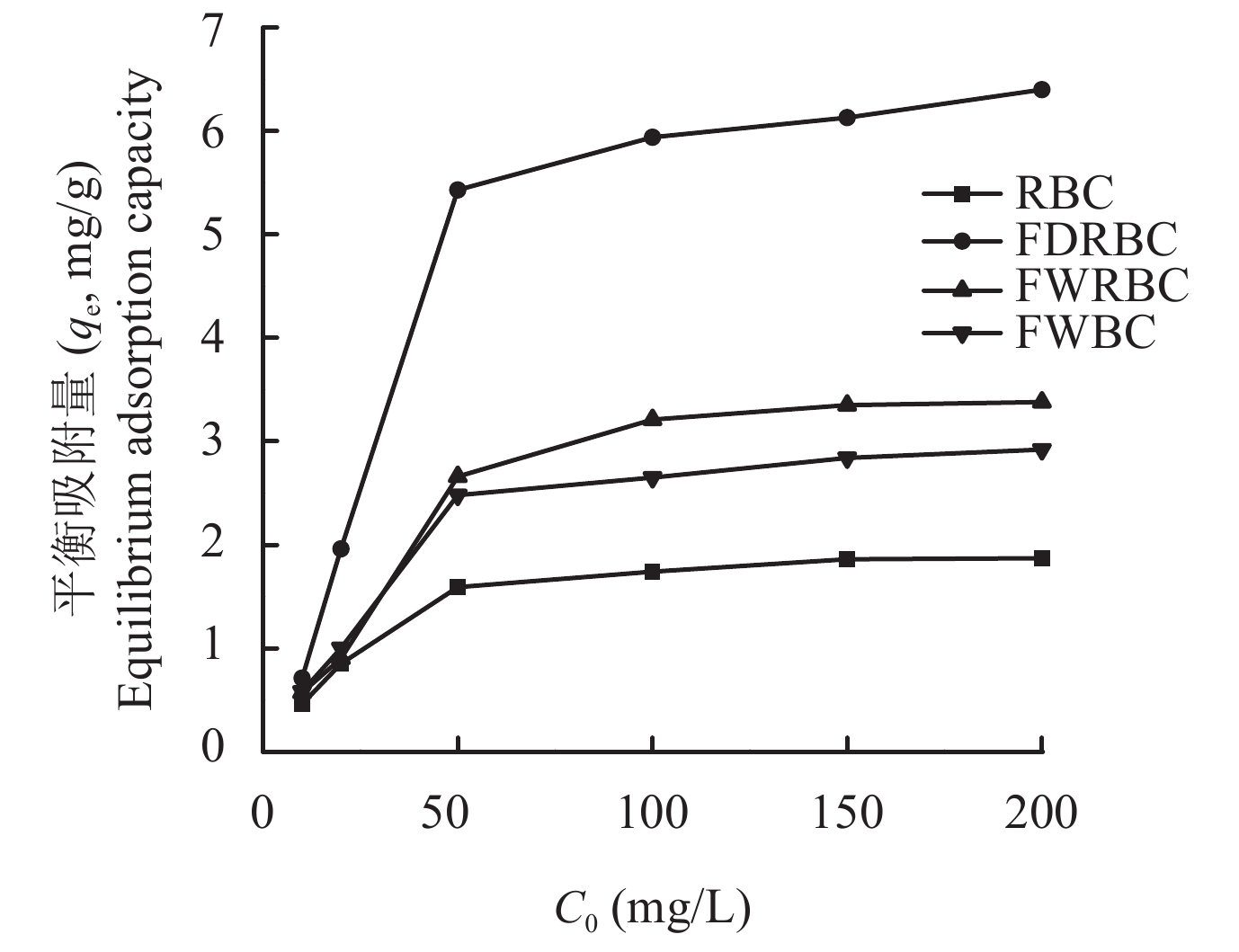
图8显示,在铵态氮初始溶液浓度较低时稻壳炭和3种铁改性生物炭对NH4+-N的吸附效果随溶液浓度的增加而增加,之后趋于平衡。储备液浓度低于50 mg/L时,稻壳炭对铵态氮的吸附增长速率最低,当储备液浓度达到50 mg/L时,稻壳炭对NH4+-N的吸附达到平衡。FDRBC对铵态氮吸附的增长速率最高,而且当储备液浓度为200 mg/L时,其吸附量仍有一定的上升趋势,表明其吸附位点还未达到饱和状态。FWRBC在储备液浓度为100 mg/L时对铵态氮的吸附达到平衡状态,FWBC对铵态氮的吸附量低于FWRBC,但是当储备液浓度为200 mg/L时,其吸附量仍有上升趋势,表明其吸附效果还有提升的空间。由于制备工艺不同,几种改性生物炭在不同的初始浓度下对NH4+-N的吸附量表现出较大差异,总体来说吸附量表现为FDRBC > FWRBC > FWBC > RBC。
![]() 图 8 初始浓度对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 8. Effects of initial concentration on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 8 初始浓度对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 8. Effects of initial concentration on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar2.5 生物炭对NH4+的吸附等温线
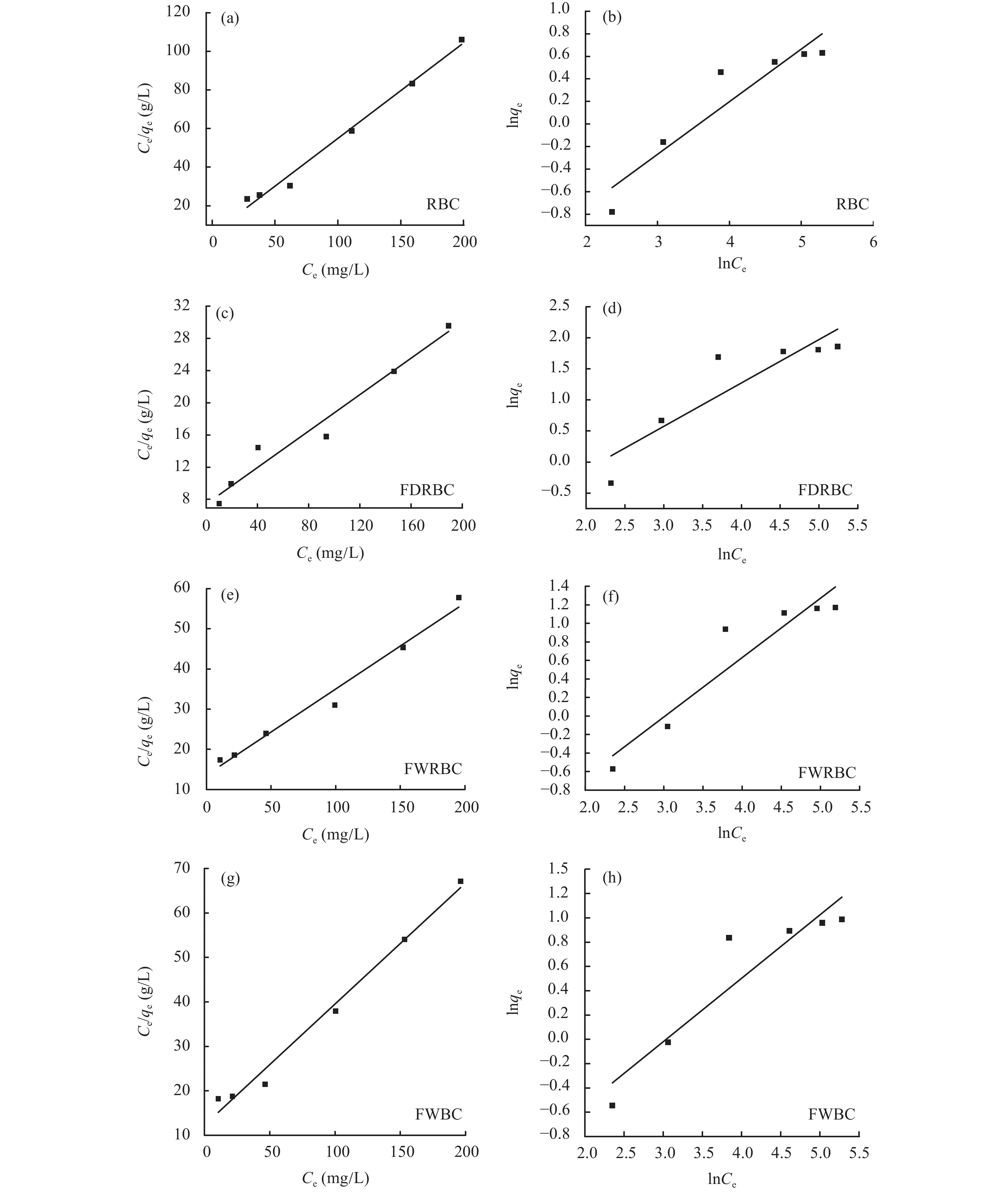
等温线拟合结果如图9所示,方程拟合参数如表2所示。分析比较两个方程拟合等温吸附模型参数可以得到,稻壳炭和3种铁改性生物炭的Langmuir吸附等温方程的R2分别为0.987、0.955、0.977、0.984,较Freundlich吸附等温方程拟合效果更好,表明稻壳炭和3种铁改性生物炭对铵态氮的吸附行为主要为单分子层吸附。Langmuir方程中的常数KL可表征吸附剂和吸附质的结合性能,KL越大表示吸附结合的稳定性越强,稻壳炭的KL最大为0.031,其次是FWBC为0.022,FDRBC和FWRBC的KL分别为0.015和0.016,表明稻壳炭吸附铵态氮的稳定性最高。RBC、FDRBC、FWRBC和FWBC的最大吸附量 (qmax) 分别为2.22、8.82、4.67、3.67 mg/g,这与稻壳炭和3种铁改性生物炭对铵态氮吸附的实际测量值也较为接近。
![]() 图 9 稻壳炭和3种铁改性稻壳炭吸附铵态氮的 Langmuir 吸附等温线 (a、c、e、g) 与Freundlich 吸附等温线 (b、d、f、h)[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 9. Langmuir (a, c, e, g) and Freundlich (b, d, f, h) isotherm of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar表 2 等温吸附模型拟合参数Table 2. Simulation of isothermal adsorption model and corresponding parameters
图 9 稻壳炭和3种铁改性稻壳炭吸附铵态氮的 Langmuir 吸附等温线 (a、c、e、g) 与Freundlich 吸附等温线 (b、d、f、h)[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 9. Langmuir (a, c, e, g) and Freundlich (b, d, f, h) isotherm of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar表 2 等温吸附模型拟合参数Table 2. Simulation of isothermal adsorption model and corresponding parameters样品
SampleLangmuir方程 Langmuir function Freundlich方程 Freundlich function KL qmax R2 KF n R2 RBC 0.031 2.224 0.987 0.171 2.141 0.855 FDRBC 0.015 8.820 0.955 0.218 1.143 0.777 FWRBC 0.016 4.671 0.977 0.145 1.560 0.867 FWBC 0.022 3.674 0.984 0.192 1.812 0.847 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method. 2.6 生物炭对NH4+的吸附动力学
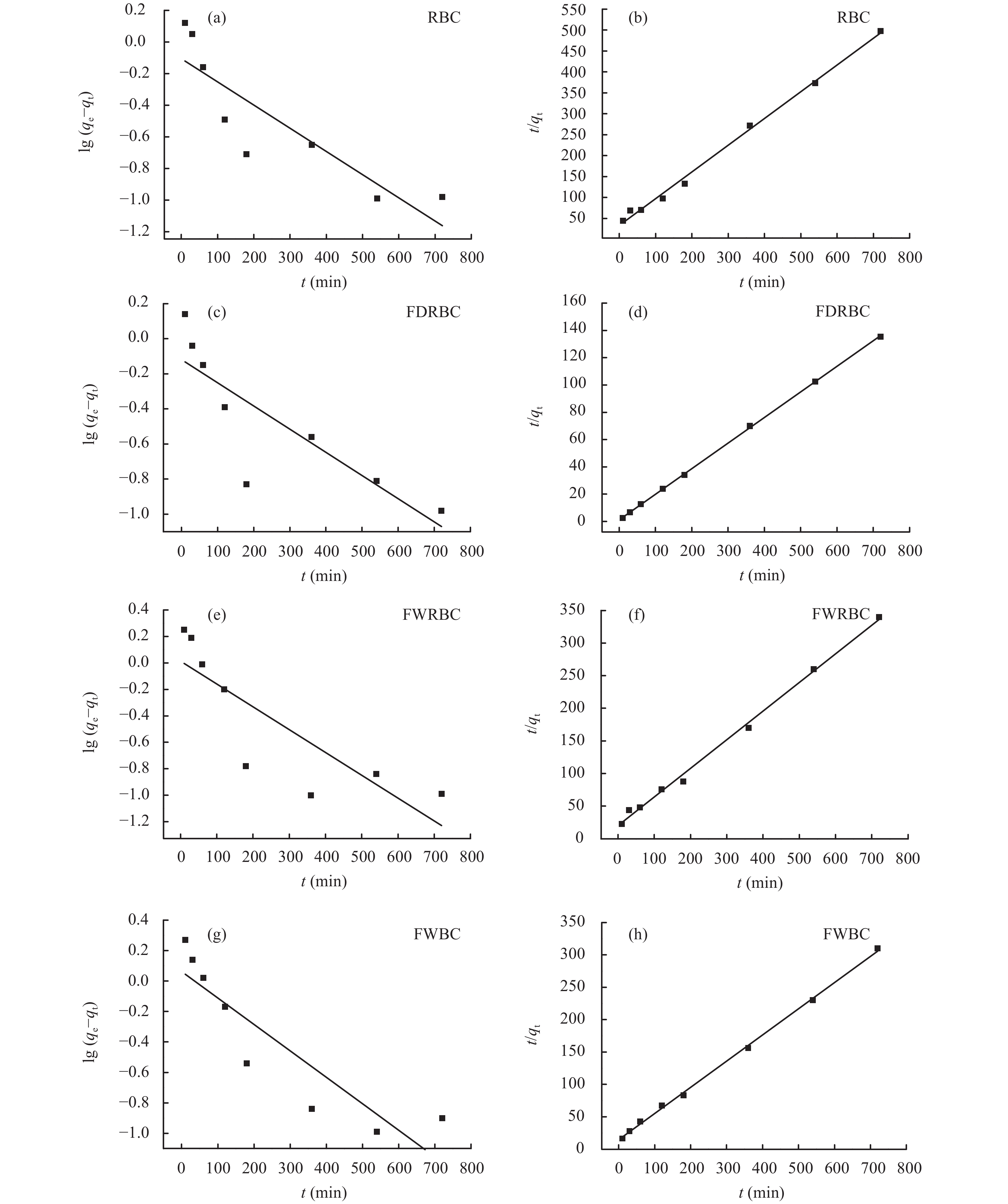
为了更好地分析稻壳炭和3种铁改性稻壳炭对铵态氮吸附的动力学行为,采用准一级动力学方程和准二级动力学方程对吸附数据进行拟合 (图10),方程拟合参数如表3所示。RBC、FDRBC、FWRBC和FWBC对铵态氮的吸附行为以准一级动力学方程拟合效果较差,其吸附行为以准二级动力学方程更为符合,R2分别为0.995、0.999、0.996、0.998,其中铁改性稻壳炭的拟合效果优于稻壳炭,FDRBC对铵态氮的吸附行为以准二级动力学方程的拟合效果最好。并且用准二级动力学方程拟合得到的稻壳炭和3种铁改性稻壳炭对铵态氮的吸附量与实测值也较为接近。k2值代表吸附速率,稻壳炭的k2值在4种生物炭中最低,表明铁改性稻壳炭对铵态氮的吸附速率高于稻壳炭。
![]() 图 10 稻壳炭和3种铁改性稻壳炭吸附铵态氮的准一级 (a、c、e、g) 和准二级 (b、d、f、h) 动力学模型[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 10. Pseudo-first order kinetics sorption (a, c, e, g) and pseudo-second order kinetics sorption models (b, d, f, h) of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar表 3 准一级动力学模型和准二级动力学模型拟合参数Table 3. Kinetics parameters based on pseudo-first-order and pseudo-second-order kinetics models
图 10 稻壳炭和3种铁改性稻壳炭吸附铵态氮的准一级 (a、c、e、g) 和准二级 (b、d、f、h) 动力学模型[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]Figure 10. Pseudo-first order kinetics sorption (a, c, e, g) and pseudo-second order kinetics sorption models (b, d, f, h) of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar表 3 准一级动力学模型和准二级动力学模型拟合参数Table 3. Kinetics parameters based on pseudo-first-order and pseudo-second-order kinetics models样品
Sample试验参数 Experimental parameter 准一级动力学方程 Pseudo-first order 准二级动力学方程 Pseudo-second order pH qe,exp (mg/g) k1 (min–1) qe,cal (mg/g) R2 k2 [g/(mg·min)] qe,cal (mg/g) R2 RBC 7 1.55 0.0034 0.784 0.738 0.0122 1.566 0.995 FDRBC 7 5.42 0.0030 0.761 0.666 0.0301 5.335 0.999 FWRBC 7 2.22 0.0040 1.030 0.666 0.0195 2.279 0.996 FWBC 7 2.45 0.0040 0.867 0.784 0.1113 2.474 0.998 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.] 3. 讨论
3.1 生物炭的表征
经过铁改性后生物炭的理化特性有了显著的改变,FDRBC相较于稻壳炭比表面积显著减小,平均孔径显著增加。其原因可能是铁改性在生物炭表面负载的金属或金属氧化物分布不均,致使稻壳炭原本有序的空间结构遭到破坏,从而降低了稻壳炭的比表面积,但也因此使得生物炭的小孔被打通,中孔和大孔得以生成,孔径显著提高[13,17]。虽然经铁改性后FDRBC的BET比表面积降低,但是孔径和总孔容积明显提高,生物炭表面还负载了Fe3O4,对铵态氮的吸附效果依然有明显提高。本试验中稻壳炭pH呈碱性,这是由于生物炭含有较多的盐基离子和碱性基团[18]。而3种铁改性生物炭均呈酸性,这可能是由于改性过程中添加了FeCl3,FeCl3为强酸弱碱盐,在水解过程中会消耗一部分的OH–,电离出H+。经铁改性后生物炭碳含量均有所降低,这可能是由于FeCl3和生物质的热性能不同,影响了热解过程中的碳化过程,极大地改变了生物炭孔隙的形成和碳的保存[19]。从X射线衍射分析图谱来看,干式混合热解法对生物炭晶体结构的改变最为显著。FDRBC的X射线衍射分析图谱生成了较多的衍射峰,主要是由于FeCl3的加入,在热解过程中形成较多的FeO结晶,并且在热解过程中γ-Fe2O3晶体会转变为α-Fe2O3晶体,但α-Fe2O3晶体热稳定性较弱,会导致晶体孔隙结构破坏,使衍射峰增多[20]。
3.2 pH对生物炭吸附NH4+的影响
溶液的PH对生物炭吸附效果也有明显的影响,溶液pH较低时稻壳炭表面呈正电荷,与NH4+存在静电斥力作用,不易于吸附[21],同时酸性条件下,溶液中存在的H+还会与NH4+-N竞争吸附位点,进而影响NH4+-N的吸附[22]。此外铁改性生物炭在铵态氮溶液中还会发生如下的反应关系:
{\rm{ \text{—} Fe \text{—} CO}}{{\rm{H}}_{\left( {\rm{s}} \right)}}{\rm{ + }}{{\rm{H}}^{\rm{ + }}}<=>{\rm{Fe \text{—} CO}}{{\rm{H}}_{\rm{2}}}_{\left( {\rm{s}} \right)}^{\rm{ + }} (8) {\rm{ \text{—} Fe \text{—} CO}}{{\rm{H}}_{\left( {\rm{s}} \right)}}<=>{\rm{ Fe \text{—} C}}{{\rm{O}}^{\rm{ - }}}_{\left( {\rm{s}} \right)}{\rm{ + }}{{\rm{H}}^{\rm{ + }}} (9) 由于质子化反应—Fe—CO–(s)更加易于结合铵态氮,增强吸附效果,在低pH情况下,H+会竞争NH4+的吸附位点,使吸附容量降低;随着pH的升高,铁改性生物炭表面发生去质子化反应,暴露出吸附位点,提高吸附容量[23]。由于FDRBC负载的铁离子含量最高,因此其对铵态氮的吸附效果也强于FWRBC、FWBC和RBC。当pH过高时,稻壳炭和3种铁改性生物炭对NH4+-N的吸附效果降低。这可能是由于当pH过高时,溶液中游离氨逐渐成为氨氮的主要存在形式[3],不利于生物炭对NH4+-N的吸附[24]。并且随着pH的增大,溶液中铁改性生物炭表面往往会形成金属水合物、金属盐沉淀等固体,降低了生物炭表面基团活性,同时会堵塞生物炭孔隙,影响对铵态氮的吸附性能[25]。本试验中稻壳炭和3种铁改性稻壳炭在不同pH环境下对NH4+-N的平衡吸附量变化趋势与陈靖等[26]的试验结果相一致。
3.3 反应时间对生物炭吸附NH4+的影响
在反应初期,生物炭对铵态氮的吸附量快速增长,此时生物炭表面有大量的吸附位点,同时溶液中铵态氮浓度较高,有利于铵态氮的快速吸附[27]。随着时间的延长及吸附解吸反应的进行,稻壳炭和3种铁改性生物炭对铵态氮的吸附量增长的趋势变得缓慢,逐渐达到吸附饱和的状态。宋婷婷等[28]研究竹炭与玉米秸秆炭等对铵态氮吸附效果,其结果与本试验类似。稻壳炭和3种铁改性生物炭比较,稻壳炭最先对铵态氮达到吸附平衡状态。由表1可知,RBC芳香结构最为丰富,表明RBC表面与NH3+的阳离子-π作用最强,阳离子-π作用是存在于阳离子和芳香性结构之间的相互作用,其共轭结构越多吸附强度越高[29],因此RBC最早对铵态氮达到吸附平衡状态。
3.4 初始溶液浓度对生物炭吸附NH4+的影响
随着储备液浓度的提高,稻壳炭和3种铁改性生物炭对NH4+-N的吸附逐渐达到平衡。这是因为当NH4+-N浓度过高时,生物炭表面的吸附位点数量有限,其表面离子的各种相互作用也达到动态平衡的状态,生物炭会因静电吸附和表面吸附能力有限而无法继续进行吸附行为[30]。而铁改性稻壳炭对铵态氮吸附性能优于稻壳炭,也导致铁改性稻壳炭对铵态氮的吸附达到平衡时其铵储备液浓度更高。这可能是由于铁改性稻壳炭中负载了金属铁离子,导致生物炭表面的吸附位点增多,吸附能力增强,这与柳富杰等[31]的研究结果一致。
3.5 生物炭对NH4+的吸附等温线
本试验中,Langmuir等温方程能够更好地拟合铁改性稻壳炭和稻壳炭对铵态氮的吸附数据。这与平巍等[32]的铁改性香蕉皮生物炭对铵态氮的吸附效果研究得到了一致的结果,其吸附行为更符合Langmuir等温方程,吸附方式主要为单分子层吸附,吸附效果更好。林静雯等[33]在牛粪生物炭和玉米秸秆生物炭对铵态氮的吸附效果研究中发现,其吸附行为更符合Freundlich吸附等温线,其原因可能是吸附材料和制备方式不同。有学者研究表明,玉米秸秆炭、芦苇炭、木炭、活性炭以及稻壳炭对铵态氮的最大吸附量分别为3.93、1.49、2.19、3.77和2.04 mg/g,其吸附性能均低于本试验中干式混合热解法制备的铁改性稻壳炭FDRBC对铵态氮的最大吸附量[22, 34-35]。
3.6 生物炭对NH4+的吸附动力学
在本试验中,稻壳炭和3种铁改性稻壳炭对铵态氮的吸附行为更加符合准二级动力学方程,因此推断稻壳炭和3种铁改性稻壳炭对铵态氮的吸附行为主要为化学吸附,这也与由Langmuir等温方程推断出的稻壳炭和3种铁改性稻壳炭对铵态氮的吸附方式主要为单分子层吸附的结果相一致。该吸附行为主要分为两步:1) 吸附质到达吸附剂表面;2) 吸附质通过离子交换或者络合反应与吸附剂相结合。这与之前一些学者对玉米秸秆炭与铁改性污泥生物炭等对铵态氮的吸附性能的研究结果[36-37]相类似。
4. 结论
1) 铁改性稻壳炭的物理和化学性质均发生了显著变化。铁改性稻壳炭pH较稻壳炭显著降低,其中FDRBC的理化性质改变最为显著,FDRBC的BET比表面积显著降低,孔径和总孔容积明显提高,晶体结构变为多晶物质,提高了对铵态氮的吸附性能。
2) pH、反应时间和溶液初始浓度均会对生物炭吸附铵态氮产生影响。本试验中pH为7,反应时间为180 min,初始浓度为100 mg/L时FDRBC对铵态氮的吸附效果较好。
3) 稻壳炭和3种铁改性稻壳炭对铵态氮的吸附行为主要为单分子层化学吸附。
4) 总体而言,稻壳炭和3种铁改性稻壳炭对铵态氮的吸附能力有一定的差异,其中干式混合热解法制备的铁改性稻壳炭FDRBC对铵态氮的吸附性能显著优于稻壳炭和其他两种方式改性的稻壳炭。
-
图 1 稻壳炭和3种铁改性稻壳炭样品扫描照片
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 1. SEM images of rice husk biochar and three types of iron-modified rice husk biochar
图 2 稻壳炭和3种铁改性稻壳炭X射线衍射图
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 2. XRD pattern of rice husk biochar and three types of iron-modified rice husk biochar
图 3 稻壳炭和3种铁改性稻壳炭FT-IR光谱图
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 3. FT-IR spectra of rice husk biochar and three types of iron-modified rice husk biochar
图 4 稻壳炭和3种铁改性稻壳炭氮气吸附/脱附等温线
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 4. Nitrogen adsorption-desorption isotherms of rice husk biochar and three types of iron-modified rice husk biochar
图 5 稻壳炭和3种铁改性稻壳炭孔径分布图
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 5. Pore size distributions of rice husk biochar and three types of iron-modified rice husk biochar
图 6 pH 对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 6. Effects of pH on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 7 反应时间对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 7. Effects of contact time on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 8 初始浓度对稻壳炭和3种铁改性稻壳炭吸附铵态氮的影响
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 8. Effects of initial concentration on the adsorption of ammonium nitrogen onto rice husk biochar and three types of iron-modified rice husk biochar
图 9 稻壳炭和3种铁改性稻壳炭吸附铵态氮的 Langmuir 吸附等温线 (a、c、e、g) 与Freundlich 吸附等温线 (b、d、f、h)
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 9. Langmuir (a, c, e, g) and Freundlich (b, d, f, h) isotherm of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar
图 10 稻壳炭和3种铁改性稻壳炭吸附铵态氮的准一级 (a、c、e、g) 和准二级 (b、d、f、h) 动力学模型
[注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.]
Figure 10. Pseudo-first order kinetics sorption (a, c, e, g) and pseudo-second order kinetics sorption models (b, d, f, h) of ammonium nitrogen adsorption onto rice husk biochar and three types of iron-modified rice husk biochar
表 1 生物炭的基本理化性质
Table 1 Basic physical and chemical properties of biochar
样品
SampleBET比表面积 (m2/g)
BET specific surface area孔径 (nm)
Average pore sizepH RBC 12.4 ± 1.2 a 8.72 ± 1.6 c 10.70 ± 0.6 a FDRBC 7.0 ± 0.7 b 36.55 ± 2.8 a 4.61 ± 0.4 b FWRBC 12.1 ± 0.9 a 8.96 ± 2.4 bc 5.08 ± 0.3 b FWBC 4.5 ± 0.6 c 12.11 ± 0.9 b 5.44 ± 0.3 b 样品
SampleN (%) C (%) H (%) O (%) H/C O/C RBC 0.72 ± 0.02 a 57.73 ± 1.5 a 3.82 ± 0.05 c 7.9 ± 0.6 a 0.066 ± 0.002 c 0.137 ± 0.001 b FDRBC 0.70 ± 0.03 a 40.63 ± 3.1 c 3.91 ± 0.14 c 6.8 ± 0.4 b 0.096 ± 0.007 a 0.167 ± 0.005 a FWRBC 0.71 ± 0.05 a 51.49 ± 0.9 b 4.14 ± 0.07 b 8.4 ± 0.5 a 0.080 ± 0.003 b 0.163 ± 0.002 a FWBC 0.72 ± 0.02 a 49.68 ± 2.3 b 4.61 ± 0.05 a 8.4 ± 0.7 a 0.093 ± 0.006 a 0.169 ± 0.007 a 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method; 同列数据后不同字母表示不同处理间差异显著 (P < 0.05) Values followed by different letters in a column indicate significant difference among treatments (P < 0.05). 表 2 等温吸附模型拟合参数
Table 2 Simulation of isothermal adsorption model and corresponding parameters
样品
SampleLangmuir方程 Langmuir function Freundlich方程 Freundlich function KL qmax R2 KF n R2 RBC 0.031 2.224 0.987 0.171 2.141 0.855 FDRBC 0.015 8.820 0.955 0.218 1.143 0.777 FWRBC 0.016 4.671 0.977 0.145 1.560 0.867 FWBC 0.022 3.674 0.984 0.192 1.812 0.847 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method. 表 3 准一级动力学模型和准二级动力学模型拟合参数
Table 3 Kinetics parameters based on pseudo-first-order and pseudo-second-order kinetics models
样品
Sample试验参数 Experimental parameter 准一级动力学方程 Pseudo-first order 准二级动力学方程 Pseudo-second order pH qe,exp (mg/g) k1 (min–1) qe,cal (mg/g) R2 k2 [g/(mg·min)] qe,cal (mg/g) R2 RBC 7 1.55 0.0034 0.784 0.738 0.0122 1.566 0.995 FDRBC 7 5.42 0.0030 0.761 0.666 0.0301 5.335 0.999 FWRBC 7 2.22 0.0040 1.030 0.666 0.0195 2.279 0.996 FWBC 7 2.45 0.0040 0.867 0.784 0.1113 2.474 0.998 注(Note):RBC―稻壳炭 Rice husk biochar; FDRBC―干式混合热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by dry mixed pyrolysis method; FWRBC―湿式浸渍热解法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by wet impregnation pyrolysis method; FWBC―酸碱浸渍改性法制备的铁改性稻壳炭 Iron-modified rice husk biochar was produced by acid-base impregnation modification method.] -
[1] 黄晶, 刘立生, 马常宝, 等. 近30年中国稻区氮素平衡及氮肥偏生产力的时空变化[J]. 植物营养与肥料学报, 2020, 26(6): 987–998. Huang J, Liu L S, Ma C B, et al. Spatial-temporal variation of nitrogen balance and partial factor productivity of nitrogen in rice region of China over the past 30 years[J]. Journal of Plant Nutrition and Fertilizers, 2020, 26(6): 987–998. Hang J, Liu L S, Ma C B, et al. Spatial-temporal variation of nitrogen balance and partial factor productivity of nitrogen in rice region of China over the past 30 years[J]. Journal of Plant Nutrition and Fertilizers, 2020, 26(06): 987–998.
[2] 王士红, 聂军军, 李秋芝, 等. 施氮量对土壤–棉花系统中氮素吸收利用和氮素去向的影响[J]. 植物营养与肥料学报, 2020, 26(4): 738–745. Wang S H, Nie J J, Li Q Z, et al. Effects of nitrogen application rate on nitrogen absorption and utilization, and fate of nitrogen in soil-cotton system[J]. Journal of Plant Nutrition and Fertilizers, 2020, 26(4): 738–745. Wang S H, Nie J J, Li Q Z, et al. Effects of nitrogen application rate on nitrogen absorption and utilization, and fate of nitrogen in soil-cotton system[J]. Journal of Plant Nutrition and Fertilizers, 2020, 26(04): 738-745.
[3] Kumar V J, Shigeo H. Modification on natural clinoptilolite zeolite for its NH4+ retention capacity[J]. Journal of Hazardous Materials, 2009, 169(1–3): 29–35.
[4] 周翔. 覆膜与控/缓释肥互作对渭北旱塬春玉米生长和水氮利用的影响[D]. 陕西杨凌: 西北农林科技大学硕士学位论文, 2019: 34–35. Zhou X. Influences on spring maize growth, water and nitrogen utilization by the interactions of plastic mulching and controlled/slow release fertilizer in the Weibei Dryland[D]. Yangling, Shaanxi: MS Thesis of Northwest A&F University, 2019: 34–35.
[5] 李文涛, 柴宝华, 王美净, 等. 不同生活垃圾组分热解炭化特性与热解焦傅里叶红外光谱表征[J]. 新能源进展, 2020, 8(1): 22–27. Li W T, Chai B H, Wang M J, et al. Pyrolysis carbonization and char FTIR characterization study on different components of municipal solid waste[J]. Advances in New and Renewable Energy, 2020, 8(1): 22–27. DOI: 10.3969/j.issn.2095-560X.2020.01.004 Li W T, Chhai B H, Wang M J, et al. Pyrolysis Carbonization and Char FTIR Characterization Study on Different Components of Municipal Solid Waste[J]. Advances in New And Renewable Energy, 2020.8(01): 22–27. DOI: 10.3969/j.issn.2095-560X.2020.01.004
[6] 李星燃, 梁媛. 生物炭的13C标记方法及对镉、铜的吸附性能探究[J]. 环境科学学报, 2020, 40(8): 1–8. Li X R, Liang Y. 13C labeled biochar and its adsorption performance on cadmium and copper[J]. Acta Scientiae Circumstantiae, 2020, 40(8): 1–8. Li X R, Liang Y. 13C labeled biochar and its adsorption performance on cadmium and copper[J]. Acta Scientiae Circumstantiae, 2020, 40(8): 1–8.
[7] 何东霞, 李文涛, 柴宝华, 等. 生物有机肥部分替代化肥对韭菜生长生理及肥料利用率的影响[J]. 西北农业学报, 2020, 29(6): 958–967. He D X, Li W T, Chai B H, et al. Effect of partial substitution of chemical fertilizer with bio-organic fertilizer on growth physiological and fertilizer utilization efficiency in chinese chives[J]. Acta Agriculturae Boreali-occideutalis Sinica, 2020, 29(6): 958–967. He D X, Li W T, Chai B H, et al. Effect of partial substitution of chemical fertilizer with bio-organic fertilizer on growth physiological and fertilizer utilization efficiency in chinese chives[J]. Acta Agriculturae Boreali-occideutalis Sinica, 2020, 29(6): 958–967.
[8] Ding Y, Liu Y X, Wu W X, et al. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns[J]. Water, Air, & Soil Pollution, 2010, 213(1–4): 47–55.
[9] 刘雪梅, 万娟娟, 王宇航, 等. 不同agro–生物质炭对水土两相中铵态氮的固持效应[J]. 环境工程学报, 2016, 10(9): 5165–5171. Liu X M, Wan J J, Wang Y H, et al. Effects of different agro-biochar on ammonium nitrogen retention in soils and waters[J]. Chinese Journal of Environmental Engineering, 2016, 10(9): 5165–5171. Liu X M, Wan J J, Wang Y H, et al. Effects of different agro-biochar on ammonium nitrogen retention in soils and waters[J]. Chinese Journal of Environmental Engineering, 2016.10(09): 5165–5171.
[10] 建晓朋, 许伟, 侯兴隆, 等. 活性炭改性技术研究进展[J]. 生物质化学工程, 2020, 54(5): 66–72. Jian X P, Xu W, Hou X L, et al. Research progress on activated carbon modification technology[J]. Biomass Chemical Engineering, 2020, 54(5): 66–72. DOI: 10.3969/j.issn.1673-5854.2020.05.009 Jian X P, Xu W, Hou X L, et al. Research Progress on Activated Carbon Modification Technology[J]. Biomass Chemical Engineering, 2020, 54(05): 66–72. DOI: 10.3969/j.issn.1673-5854.2020.05.009
[11] 祝天宇, 卢泽玲, 刘月娥, 等. 镁改性生物炭制备条件对其氮、磷去除性能的影响[J]. 环境工程, 2018, 36(1): 37–41. Zhu T Y, Lu Z L, Liu Y E, et al. Effects of preparation conditions of Mg–modified biochar on the removal of ammonium and phosphate in wastewater[J]. Environmental Engineering, 2018, 36(1): 37–41. Zhu T Y, Lu Z L, Liu Y E, et al. Effects of preparation condrtions of Mg–modified biochar on the removal of ammoniumand phosphate in wastewater[J]. Environmental Engineering, 2018, 36(1): 37–41.
[12] 王思源, 申健, 李盟军, 等. 不同改性生物炭功能结构特征及其对铵氮吸附的影响[J]. 生态环境学报, 2019, 28(5): 1037–1045. Wang S Y, Shen J, Li M J, et al. Functional and structural characteristics of different modified biochar and its impacts on ammonium nitrogen adsorption[J]. Ecology and Environmental Sciences, 2019, 28(5): 1037–1045. Wang S Y, Shen J, Li M J, et al. Functional and structural characteristics of different modified biochar and its impacts on ammonium nitrogen adsorption[J]. Ecology and Environmental Sciences, 2019, 28(5): 1037–1045.
[13] 张文, 吕欣田, 韩睿, 等. 2种改性生物炭对水体硝态氮的吸附特性[J]. 生态与农村环境学报, 2018, 34(3): 253–259. Zhang W, Lü X T, Han R, et al. Effects of two kinds of modified biochar adsorbing nitrate-N in water[J]. Journal of Ecology and Rural Environment, 2018, 34(3): 253–259. DOI: 10.11934/j.issn.1673-4831.2018.03.009 Zhang W, Lv X T, Han R, et al. Effects of two kinds of modified biochar adsorbing nitrate-N in Water[J]. Journal of Ecology and Rural Environment, 2018.34(3): 253–259. DOI: 10.11934/j.issn.1673-4831.2018.03.009
[14] 智燕彩, 赖欣, 谭炳昌, 等. 铁锰镁离子改性生物炭对溶液硝态氮的吸附性能研究[J]. 核农学报, 2020, 34(7): 1588–1597. Zhi Y C, Lai X, Tan B C, et al. Adsorption of nitrate by iron, manganese and magnesium ion modified biochars[J]. Journal of Nuclear Agricultural Sciences, 2020, 34(7): 1588–1597. DOI: 10.11869/j.issn.100-8551.2020.07.1588 Zhi Y C, Lai X, Tan B C, et al. Adsorption of nitrate by iron, manganese and magnesium ion modified biochars[J]. Journal of Nuclear Agricultural Sciences, 2020, 34(7): 1588–1597. DOI: 10.11869/j.issn.100-8551.2020.07.1588
[15] 康宁, 毛磊, 张宇, 等. 负载铁生物炭的制备、表征及其对Cr(Ⅵ)的吸附性能[J]. 武汉科技大学学报, 2020, 43(3): 185–193. Kang N, Mao L, Zhang Y, et al. Preparation, characterization and adsorption for Cr(Ⅵ) of Fe-impregnated biochar[J]. Journal of Wuhan University of Science and Technology, 2020, 43(3): 185–193. Kang N, Mao L, Zhang Y, et al. Preparation, chavacterization and adsorption for Cr(Ⅵ) of Fe-impregnated biochar[J]. Journal of Wuhan University of Science and Technology, 2020, 43(03): 185–193.
[16] 陈锋, 赵一静, 孙哲, 等. 豆渣派生微介孔碳吸附橙黄G的动力学和热力学[J]. 水处理技术, 2020, 46(8): 1–7. Chen F, Zhao Y J, Sun Z, et al. Adsorption kinetics and thermodynamics of orange G by micro-mesoporous carbon derived from soybean residue[J]. Technology of Water Treatment, 2020, 46(8): 1–7. Chen F, Zhao Y J, Sun Z, et al. Adsorption kinetics and thermodynamics of orange g by micro-mesoporous carbon derived from soybean residue[J]. Technology of Water Treatment, 2020, 46(08): 1–7.
[17] 陈靖. Fe/Mg改性生物炭去除水中氮磷的研究[D]. 重庆: 重庆大学硕士学位论文, 2015: 51–53. Chen J. The study on removing ammonia nitrogen and phosphorus in water by Fe/Mg-modified bamboo charcoal[D]. Chongqing: MS Thesis of Chongqing University, 2015: 51–53.
[18] 赵越, 赵保卫, 刘辉, 等. 热解温度对生物炭理化性质和吸湿性的影响[J]. 环境化学, 2020, 39(7): 1–8. Zhao Y, Zhao B W, Liu H, et al. Effect of pyprolysis temperature on physicochemical properties and hygroscopicity of bichar[J]. Environmental Chemistry, 2020, 39(7): 1–8. Zhao Y, Zhao B W, Liu H, et al. Effect of pyprolysis temperature on physicochemical properties and hygroscopicity of bichar[J]. Environmental Chemistry, 2020, 39(7): 1–8.
[19] Li R, Jim J, Wang B Y, et al. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment[J]. Journal of Cleaner Production, 2017, 147: 96–107. DOI: 10.1016/j.jclepro.2017.01.069
[20] 周飞, 熊志波, 金晶, 等. 煅烧温度对磁性铁钛复合氧化物微观结构及脱硝活性的影响[J]. 化工进展, 2018, 37(9): 3410–3415. Zhou F, Xiong Z B, Jin J, et al. Influence of calcination temperature on the micro-structure and the NH3-SCR activity of magnetic iron-titanium mixed oxide catalyst[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3410–3415. Zhou F, Xiong Z B, Jin J, et al. Influence of calcination temperature on the micro-structure and the NH3-SCR activity of magnetic iron-titanium mixed oxide catalyst[J]. Chemical Industry and Engineering Progress, 2018.37(09): 3410–3415.
[21] 彭启超, 刘小华, 罗培宇, 等. 不同原料生物炭对氮、磷、钾的吸附和解吸特性[J]. 植物营养与肥料学报, 2019, 25(10): 1736–1772. Peng Q C, Liu X H, Luo P Y, et al. Adsorption and desorption characteristics of nitrogen, phosphorus and potassium by biochars from different raw materials[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(10): 1736–1772. Peng Q C, Liu X H, Luo P Y, et al. Adsorption and desorption characteristics of nitrogen, phosphorus and potassium by biochars from different raw materials[J]. Journal of Plant Nutrition and Fertilizers, 2019.25(10): 1736–1772.
[22] Zhu K, Hao F, Zhang J H, et al. Studies on removal of NH4 +-N from aqueous solution by using the activated carbons derived from rice husk[J]. Biomass and Bioenergy, 2012, 43(8): 18–25.
[23] Oeztuerk N, Bektas T E. Nitrate removal from aqueous solution by adsorption onto various materials[J]. Journal of Hazardous Materials, 2004, 112(1/2): 155–162.
[24] 蒋旭涛. 生物炭对水中氨氮和磷酸盐吸附性能研究[D]. 天津: 天津大学硕士学位论文, 2013. 22–36. Jiang X T. Study on the adsorption of ammonia and phosphate by biochar[D]. Tianjin: MS Thesis of Tianjin University, 2013.22–36.
[25] Ren J, Nan L, Lin Z, et al. Pretreatment of raw biochar and phosphate removal performance of modified granular iron/biochar[J]. Transactions of Tianjin University, 2017, 23(4): 340–350. DOI: 10.1007/s12209-017-0064-z
[26] 陈靖, 李伟民, 丁文川, 等. Fe/Mg负载改性竹炭去除水中的氨氮[J]. 环境工程学报, 2015, 9(11): 5187–5192. Chen J, Li W M, Ding W C, et al. Removal of ammonia nitrogen by Fe/Mg-modified bamboo charcoal[J]. Chinese Journal of Environmental Engineering, 2015, 9(11): 5187–5192. DOI: 10.12030/j.cjee.20151109 Chen J, Li W M, Ding W C, et al. Removal of Ammonia Nitrogen by Fe/Mg–modified Bamboo Charcoal[J]. Chinese Journal of Environmental Engineering, 2015.9(11): 5187–5192. DOI: 10.12030/j.cjee.20151109
[27] 张聪智, 苏亚拉图, 赖欣, 等. 花生壳生物炭对铵态氮的吸附性能研究[J]. 中国农学通报, 2015, 31(33): 214–220. Zhang C Z, Soyolt, Lai X, et al. Study on adsorption of ammonium nitrogen by peanut shell biochar[J]. Chinese Agricultural Science Bulletin, 2015, 31(33): 214–220. DOI: 10.11924/j.issn.1000-6850.casb15060035 Zhang C Z, Soyolt, Lai X, et al. Study on Adsorption of Ammonium Nitrogen by Peanut Shell Biochar[J]. Chinese Agricultural Science Bulletin, 2015.31(33): 214–220. DOI: 10.11924/j.issn.1000-6850.casb15060035
[28] 宋婷婷, 赖欣, 王知文, 等. 不同原料生物炭对铵态氮的吸附性能研究[J]. 农业环境科学学报, 2018, 37(3): 576–584. Song T T, Lai X, Wang Z W, et al. Adsorption of ammonium nitrogen by biochars produced from different biomasses[J]. Journal of Agro-Environment Science, 2018, 37(3): 576–584. Song T T, Lai X, Wang Z W, et al. Adsorption of ammonium nitrogen by biochars produced from different biomasses[J]. Journal of Agro-Environment Science, 2018.37(03): 576–584.
[29] 刘健. 富含芳香环的碳基材料表面水与离子的吸附和动力学行为的理论研究[D]. 北京: 中国科学院大学博士学位论文, 2015. 46–48. Liu J. Adsorption and dynamics of water and ions on carbon–based surfaces with aromatic rings: A theoretical study[D]. Beijing: PhD Dissertation of University of Chinese Academy of Sciences, 2015. 46–48.
[30] Li M, Zhang Z S, Li Z, et al. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar[J]. Ecological Engineering, 2020, 149: 105792.
[31] 柳富杰, 周永升, 梁婷钰, 等. 氯化铁改性甘蔗渣炭吸附氨态氮的研究[J]. 化工技术与开发, 2019, 48(12): 53–57. Liu F J, Zhou Y S, Liang T Y, et al. study on adsorption of ammonia nitrogen by ferric chloride modified bagasse carbon[J]. Technology & Development of Chemical Industry, 2019, 48(12): 53–57. Liu F J, Zhou Y S, Liang T Y, et al, study on adsorption of ammonia nitrogen by ferric chloride modified bagasse carbon[J]. Technology & Development of Chemical Industry, 2019, 48(12): 53–57.
[32] 平巍, 李波, 董建丰, 等. 生物质改性吸附材料的制备工艺优化及对氨氮的吸附特性[J]. 农业环境科学学报, 2017, 36(12): 2521–2526. Ping W, Li B, Dong J F, et al. Optimization of preparation technology for biomass-modified adsorption material and its adsorption properties for ammonia nitrogen[J]. Journal of Agro-Environment Science, 2017, 36(12): 2521–2526. DOI: 10.11654/jaes.2017-0731 Ping W, Li B, Dong J F, et al. Optimization of preparation technology for biomass–modified adsorption material and its adsorption proper-ties for ammonia nitrogen[J]. Journal of Agro-Environment Science, 2017.36(12): 2521–2526. DOI: 10.11654/jaes.2017-0731
[33] 林静雯, 吴丹, 孙丽娜, 等. 牛粪生物炭吸附水中NH4+的影响因素及特性[J]. 沈阳大学学报(自然科学版), 2016, 28(3): 186–190. Lin J W, Wu D, Sun L N, et al. Characteristics of ammonia nitrogen adsorption onto biochars derived from dairy manare and its influencing factors[J]. Journal of Shengyang University (Natural Science Edition), 2016, 28(3): 186–190. Lin J W, Wu D, Sun L N, et al. Characteristics ammonia nitrogen adsorption onto biochars derived from dairy manare and its influencing factors[J]. Journal of Shengyang University (Natural Science), 2016, 28(3): 186–190.
[34] 张登宇, 刘方, 陈思琳, 等. 生物质炭对垃圾渗滤液中氨氮去除效果的研究[J]. 中国农学通报, 2012, 28(14): 264–268. Zhang D Y, Liu F, Chen S L, et al. The research of removing ammonia nitrogen in land fill leachate by biomass carbon[J]. Chinese Agricultural Science Bulletin, 2012, 28(14): 264–268. DOI: 10.3969/j.issn.1000-6850.2012.14.052 Zhang D Y, Liu F, Chen S L, et al. The research of removing ammonia nitrogen in land fill leachate by biomass carbon[J]. Chinese Agricultural Science Bulletin, 2012.28(14): 264–268. DOI: 10.3969/j.issn.1000-6850.2012.14.052
[35] Hou J, Huang L, Yang Z M, et al. Adsorption of ammonium on biochar prepared from giant reed[J]. Environmental Science and Pollution Research, 2016, 23(19): 19107–19115. DOI: 10.1007/s11356-016-7084-4
[36] 彭启超, 刘小华, 罗培宇, 等. 不同原料生物炭对氮、磷、钾的吸附和解吸特性[J]. 植物营养与肥料学报, 2019, 25(10): 1763–1772. Peng Q C, Liu X H, Luo P Y, et al. Adsorption and desorption characteristics of nitrogen, phosphorus and potassium by biochars from different raw materials[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(10): 1763–1772. DOI: 10.11674/zwyf.18406 Peng Q C, Liu X H, Luo P Y, et al. Adsorption and desorption characteristics of nitrogen, phosphorus and potassium by biochars from different raw materials[J]. Journal of Plant Natrition and Fertilizers, 2019, 25(10): 1763–1772. DOI: 10.11674/zwyf.18406
[37] 赵爽. 给水厂污泥改性活性炭去除硝酸盐氮的性能研究[D]. 北京: 北京建筑大学硕士学位论文, 2020. 43–48. Zhao S. Study on the performance of water treatment residuals modified granular activated carbon to remove nitrate[D]. Beijing: MS Thesis of Beijing University of Civil Engineering and Architecture, 2020. 43–48.
-
期刊类型引用(20)
1. 史广宇,吴贝贝,胡嘉源,施维林. 施加生物炭缓解土壤氮流失机理的研究进展. 环境化学. 2025(01): 149-163 .  百度学术
百度学术
2. 杨佳芝,杨帆,钟艳霞,贺婧,王世全. 乙撑胺改性生物炭螯合金属材料的磷吸附性能及解吸特性. 环境化学. 2025(02): 605-616 .  百度学术
百度学术
3. 衣本骥,刘畅,韩宏伟,张丰,陈涛涛,孟军,迟道才. 不同改性生物炭对稻田氮素损失及产量的影响. 中国环境科学. 2024(09): 5122-5133 .  百度学术
百度学术
4. 苏杨,冼卓慧,张俊涛. 铁改性生物炭制备及其对磷的吸附和有效性. 植物营养与肥料学报. 2024(09): 1782-1793 .  本站查看
本站查看
5. 王峰,常云妮,吕永铭,陈玉真,孙君,吴志丹,余文权. 田间老化生物质炭对茶园土壤氮素形态和细菌群落的影响. 茶叶学报. 2024(04): 33-43 .  百度学术
百度学术
6. 肖小兰,潘亭羽,王雄敏,傅鑫坤,杨天硕,阮文权. MgCl_2改性制备酒糟稻壳生物炭及对沼液氨氮吸附性能研究. 环境科学与技术. 2024(12): 33-40 .  百度学术
百度学术
7. 王港,陈丹,李江舟,张翠萍,纳红艳,代快,杨肖娥. 铁基生物炭海绵与沉水植物协同净化水体中的氮磷. 农业环境科学学报. 2023(02): 424-433 .  百度学术
百度学术
8. 陈文静,石峻岭,李雪婷,张李金,刘富强,陈正祝,庞维海,杨殿海. 纳米零价铁改性生物炭对水中氨氮的吸附特性及机制. 环境科学. 2023(06): 3270-3277 .  百度学术
百度学术
9. 杨婷婷,黄艳艳,柳维扬,张婧旻,杨红竹,李建宏,林清火. 三种改性小麦秸秆生物炭表征及其对Cu~(2+)的吸附性能. 农业工程学报. 2023(08): 222-230 .  百度学术
百度学术
10. 姜梓渔. 生物炭对花生生长发育及土壤肥力的影响. 花生学报. 2023(02): 14-21 .  百度学术
百度学术
11. 顾芳宁,韩守新,王瀚标,王玉军,马秀兰,李莉. 添加改性生物炭对黑土氮素吸附及淋溶特性的影响. 西北农林科技大学学报(自然科学版). 2023(10): 97-106+117 .  百度学术
百度学术
12. 朱玉洁,林玲,唐光木,张云舒,徐万里. 改性棉秆炭对新疆灰漠土氮肥氨挥发特征的影响. 新疆农业科学. 2023(09): 2128-2137 .  百度学术
百度学术
13. 王江南,孙晓雪,杨玲辉,姜楠,张淼,刘遵奇,赫天一. 壳聚糖、铁锰改性稻壳生物炭的表征及其Cd~(2+)吸附性能研究. 农业环境科学学报. 2023(09): 1964-1973 .  百度学术
百度学术
14. 杨家浚,余浩,王梓行,黄恩惠,任进键,关弋翔,包艳萍. 铁改性生物炭的制备及铁改性对生物炭性质的影响. 广东化工. 2023(24): 14-15+43 .  百度学术
百度学术
15. 韩宇平,袁银,王春颖,李珂,张丽璇,吴德丰. 锰离子对引黄灌区不同质地土壤铵态氮吸附影响. 河南师范大学学报(自然科学版). 2022(03): 1-9+165 .  百度学术
百度学术
16. 王思展,康海生,姚增玉. 甲醛交联板栗壳色素树脂吸附水中Ni(Ⅱ)的研究. 陕西林业科技. 2022(01): 1-6 .  百度学术
百度学术
17. 刘晶晶. 土壤中铵态氮测定方法探讨. 当代化工. 2022(06): 1509-1512 .  百度学术
百度学术
18. 吴行,王秀斌,郑琴,张帅,成宇阳,王林轩. 水滑石改性生物炭有效提高设施菜田土壤磷的吸附性能. 植物营养与肥料学报. 2022(09): 1652-1663 .  本站查看
本站查看
19. 纪文婕,孙一迪,韩正砥,谢基淦,周明耀. 纳米生物炭对铵态氮的吸附-解吸效果研究. 节水灌溉. 2022(12): 92-97+102 .  百度学术
百度学术
20. 吴奇,谭美涛,迟道才. 生物炭吸附富营养化水体氮、磷的研究进展. 沈阳农业大学学报. 2022(05): 620-629 .  百度学术
百度学术
其他类型引用(17)



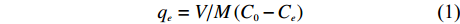
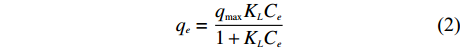
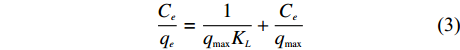
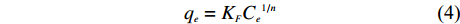
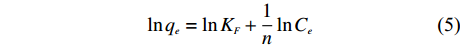
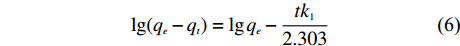
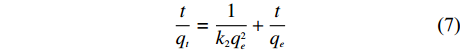
 下载:
下载: