Effect of sodium nitroprusside addition on carbon and nitrogen metabolism of rice during germination under alkali stress
-
摘要:目的
施加外源硝普纳 (sodium nitroprusside, SNP)可有效缓解水稻碱胁迫伤害。本研究通过比较不同碱敏感性水稻品种萌发及碳氮代谢相关指标的差异,探讨碱胁迫下外源SNP添加对萌发期水稻碳氮代谢的影响,为盐碱地水稻种植提供参考。
方法以碱敏感品种中花11 (ZH11)和耐碱品种宁粳52 (NG52)为试验材料,在混合碱胁迫 [alkali stress (AS), 即20 mmol/L CO32−和HCO3−等比例混合液, pH 10.50]下,设置不同SNP浓度 (0、10、30、50、70、100 μmol/L)浸种处理,通过测定萌发期关键生长指标,以筛选SNP最适浓度。结果显示碱胁迫下不同浓度外源SNP影响了水稻萌发,其中,30 μmol/L SNP处理显著增加了水稻的发芽指数,促进了水稻萌发。进一步设置CK (control)、SNP (30 μmol/L)、AS (20 mmol/L)、AS+SNP (20 mmol/L AS + 30 μmol/L SNP) 4个处理,分析它们对萌发期碱敏感差异水稻碳氮代谢的影响。
结果1) 在碳代谢方面,与AS处理相比,AS+SNP处理下ZH11和NG52中蔗糖含量显著降低。其中,NG52中蔗糖含量显著低于ZH11,而其蔗糖合成基因 (OsNIN1)和葡萄糖合成基因 (OsSPS1、OsSPS11)的表达量显著高于ZH11。2)在氮代谢方面,与AS处理相比,AS+SNP处理显著增加了2个品种中苹果酸、柠檬酸、一氧化氮含量,硝酸还原酶、S-亚硝基谷胱甘肽还原酶活性以及OsNR2、OsGSNOR1基因表达,降低了NO3−和氧化型谷胱甘肽含量,提高了脯氨酸水平。其中,NG52中苹果酸、柠檬酸、一氧化氮、脯氨酸、氧化型谷胱甘肽含量,S-亚硝基谷胱甘肽还原酶活性以及OsNR1.2、OsGSNOR1的表达量均显著高于ZH11。3)相关分析和主成分分析结果显示,葡萄糖和蔗糖合成酶基因 (OsNIN1)分别与苹果酸、柠檬酸、脯氨酸和一氧化氮呈显著正相关,葡萄糖、果糖、一氧化氮、亚硝基谷胱甘肽和氧化型谷胱甘肽可能是影响碳氮代谢相互协调的关键指标。
结论施加30 μmol/L SNP可促进碱胁迫下水稻体内蔗糖分解和有机酸的积累,增强硝酸还原酶和S-亚硝基谷胱甘肽还原酶活性,提高有机氮含量,促进碳代谢向氮代谢转化,维持碱胁迫下萌发期水稻碳氮代谢的正常进行。
Abstract:ObjectivesThe application of exogenous sodium nitroprusside (SNP) can effectively alleviate alkali stress-induced damage in rice. We observed the effect differences of exogenous SNP on the carbon and nitrogen metabolism of rice cultivars when subjecting to alkali stress at germination satge.
MethodsAlkali sensitive rice cultivar Zhonghua 11 (ZH11) and alkali resistant cultivar Ninggeng 52 (NG52) was chosed as test materials in a pot experiment. Firstly, rice seeds were soaked into alkali stress solutions (containing 20 mmol/L CO32− and HCO3−, pH 10.50) containing SNP 10, 30, 50, 70, 100 μmol/L, respectively. SNP 30 μmol/L treatment was recorded the highest germination index, so was used as the SNP concentrations for the following experiment. In the following experiment, normal nutrient solution control ( CK), normal solution containing SNP 30 μmol/L (SNP), alkali stress solution (AS), and alkali stress solution containing NSP 30 μmol/L (AS+SNP). The rice seeds were watered with the treatment solution, and the carbon and nitrogen metabolism of rice seedlings were measured.
ResultsAS+SNP treatment were recorded significantly lower sucrose content than AS treatment in both cultivar ZH11 and NG52. The sucrose contents of NG52 were significantly lower than that of ZH11, however, the expression of genes involved in sucrose synthesis (OsNIN1) and glucose synthesis (OsSPS1 and OsSPS11) in NG52 were significantly higher than that in ZH11. Compared with AS, the AS+SNP treatment markedly increased the levels of malic acid, citric acid, nitric oxide (NO), and proline content, as well as the activities of nitrate reductase (NR) and S-nitroso glutathione reductase (GSNOR) and expression of OsNR2 and OsGSNOR1, decreased the levels of NO3− and glutathione (oxidized glutathione, GSSG) in both cultivars. Furthermore, the contents of malic acid, citric acid, NO, proline, GSSG, together with GSNOR activity and the expression levels of OsNR1.2 and OsGSNOR1 were significantly higher in NG52 compared with ZH11. Positive correlations (P<0.05) were existed between genes involved in glucose and sucrose synthesis (OsNIN1) with malic acid, citric acid, proline, and NO. The principal component analysis indicated that glucose, fructose, NO, GSNO, and GSSG were key factors affecting the coordination of carbon and nitrogen metabolism.
ConclusionsTreating rice seeds with 30 μmol/L SNP solution is effective in increasing sucrose breakdown, organic acid accumulations, NR and GSNOR activities, organic nitrogen levels, and stimulating the transformation of carbon metabolism to nitrogen metabolism, thereby maintaining the normal carbon and nitrogen metabolism of rice under alkali stress during germination.
-
Keywords:
- rice /
- germination /
- alkali stress /
- sodium nitroprusside /
- carbon and nitrogen metabolism
-
土壤盐碱化是一个全球性问题。中国的盐碱地面积占世界第三,主要分布在东北、华北、西北等地区,严重制约着我国农业发展[1]。研究表明,种植水稻(Oryza sativa L.)有利于淡化土壤表层盐碱度,改善土壤结构,进而达到改良盐碱地的目的[2]。然而,水稻是一种盐碱敏感作物,种子萌发阶段易受盐碱胁迫的危害,这对幼苗及后期生长会造成不利影响[3]。例如,中性盐(NaCl、Na2SO4)与碱性盐的混合胁迫(Na2CO3、NaHCO3)显著抑制了水稻种子发芽,当混合溶液浓度达200 mmol/L时几乎完全抑制了水稻种子的发芽[4]。逆境胁迫下外施外源硝普纳(SNP)能够影响植物体内的碳氮代谢,从而使植物做出对逆境的响应和适应。如盐胁迫下施加外源0.15 mmol/L SNP、0.15 mmol/L SNP+50 µmol/L葡萄糖进行浸种处理,增加了水稻种子萌发过程中内源葡萄糖和果糖的积累,提高了盐胁迫下水稻种子早期发芽率和发芽指数,这说明糖信号分子葡萄糖通过参与调控碳代谢影响了盐胁迫下水稻种子的萌发[5]。类似地,10 µmol/L SNP增加了盐胁迫下水稻叶片中的氮含量和谷氨酸脱氢酶活性(glutamate dehydrogenase, GDH),促进蔗糖和脯氨酸的积累,为盐胁迫下水稻氮代谢提供还原力[6]。
碳氮代谢是植物体内最主要的两大代谢,包括分解、转化和积累,为植物生命活动提供能量和物质,两者协同调控植物生长发育过程[7−9]。研究表明,ygl53突变体具有较高的净同化速率、电子传递通量效率、光呼吸速率和过氧化氢酶活性,以及较低的H2O2含量和氮吸收效率。该突变体中较高活性的谷氨酸合成酶(GOGAT)和谷氨酰胺合成酶(GS)有利于合成α-酮戊二酸和氨,暗示碳代谢衍生的能量和物质可能以光呼吸的形式补充氮代谢,以维持水稻正常生长发育[10]。此外,水稻生长中后期施用氮肥不仅为生长提供充足的氮素营养,也能够提高水稻叶片净光合速率,增强碳代谢能力,促进籽粒中碳氮代谢产物积累,有助于提升产量[11]。
碱胁迫造成碳元素和氮元素的同化和吸收障碍,影响植物养分的吸收和代谢,添加外源物可调控作物体内碳氮代谢,缓解作物碱胁迫伤害。吴旭红等[12]研究发现,在60 mmol/L Na2CO3碱性盐胁迫下,添加80 μmol/L的外源SNP增强了光合作用、谷氨酰胺合成酶、谷氨酸合成酶、硝酸还原酶活性和蛋白质含量,降低游离氨基酸含量,维持了碱胁迫下南瓜幼苗碳氮代谢的正常进行,进而缓解碱胁迫对南瓜幼苗的伤害。本课题组前期研究发现,不同外源物(甜菜碱、SNP和褪黑素)均能通过调节水稻幼苗形态、生理特性及基因表达的变化,从而缓解水稻碱胁迫伤害并增强水稻的耐碱性,其中,SNP的缓解效应最大[13]。然而,碱胁迫下外源SNP的添加是否影响了水稻碳氮代谢需要进一步研究。本研究以碱敏感差异水稻品种ZH11和NG52为材料,研究碱胁迫下添加外源SNP对萌发期水稻中碳氮代谢物含量、合成酶活性及相应基因表达水平的影响,旨在探索萌发期水稻耐碱性中碳氮代谢机制,为研究SNP调控盐碱胁迫下水稻萌发和早期生长发育机理提供技术支撑和理论依据。
1. 材料与方法
1.1 试验材料
供试材料为中花11 (ZH11)和宁粳52 (NG52)。
1.2 试验设计
SNP最适浓度筛选处理包括CK (对照)、AS、AS+10 μmol/L SNP、AS+30 μmol/L SNP、AS+50 μmol/L SNP、AS+70 μmol/L SNP、AS+100 μmol/L SNP。其中,AS为混合碱溶液(NaHCO3∶Na2CO3=1∶1),其浓度20 mmol/L,pH 10.50±0.05为本课题组前期筛选碱敏感差异水稻品种时得到的最佳值[14−15]。选择两个品种的健康、饱满一致种子,经5% NaClO 消毒,蒸馏水反复冲洗后,置于不同处理的培养皿中进行萌发。每个处理是加入10 mL处理液浸种,CK是加入10 mL蒸馏水。每个处理设3个生物学重复,每个重复50粒。培养条件为 28℃/25℃、14 h/10 h (昼/夜)。所有材料经7天处理后拍照记录表型,每天对萌发相关指标进行统计。
为研究SNP对碱胁迫下不同水稻品种萌发期碳氮代谢的影响,进一步设置了4个处理:CK、30 μmol/L SNP、20 mmol/L AS、20 mmol/L AS+30 μmol/L SNP。按上述方法对不同处理下的2个品种进行萌发培养。不同处理的每个重复取3份样品,用于后续指标测定。
1.3 测定项目与方法
统计萌发第7天水稻芽长、根长、发芽数,并计算相对芽长、相对根长、发芽率和发芽指数,详细公式参照参考文献[16]。根据李合生[17]方法测定蔗糖、葡萄糖和果糖、脯氨酸、可溶性蛋白和NO3−含量;按照Hurali等[18]方法测定一氧化氮、氧化型谷胱甘肽含量和硝酸还原酶、S-亚硝基谷胱甘肽还原酶活性;采用北京索莱宝科技有限公司生产的试剂盒提取苹果酸、柠檬酸、亚硝基谷胱甘肽,提取步骤参照相应试剂盒说明书,并利用紫外分光光度法测定其OD值。
1.4 总RNA提取、cDNA合成和RT-qPCR分析
水稻幼芽进行RNA提取使用TaKaRa MiniBEST Plant RNA Extraction Kit (TaKaRa)。去除基因组DNA污染和第一链互补DNA (cDNA)合成使用PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa)。详细步骤参照使用说明书。
RT-qPCR试验根据TaKaRa公司的TB Green® Premix Ex Taq™ II试剂使用说明书进行。反应体系:TB Green Premix Ex Taq II (10 μL)、PCR Primer (1.6 μL 10 μM)、Template (cDNA 2.0 μL)和ddH2O (6.4 μL)。反应条件:预变性 (95℃ 3min);变性 (95℃ 10 s);退火 (60℃ 30 s),40个循环,熔解曲线温度从60℃改变到95℃。试验设3次重复。RT-qPCR结果采用2−ΔΔCT方法计算各基因的相对表达量[19]。根据Rice Genome Annotation Project (http://rice.uga.edu/)中的序列,利用TBtools进行RT-qPCR引物设计,内参基因为OsActin,引物信息见表1。
表 1 检测的基因和RT-qPCR引物序列Table 1. Genes examined and RT-qPCR primer sequences基因名称
Gene symbol登录号
RGAP ID引物序列 (5′—3′) (正向/反向)
Primer sequence (5′—3′) (forward/reverse)产物大小 (bp)
Product sizeOsNIN1 LOC_Os03g20020 CTATTCTGCTTTGCGTTGT/CTGAGCCTGTAGTTGATGG 87 OsNIN3 LOC_Os02g32730 TGCGGGAGTCCGTGGTTTA/GCATCATTCGGGTCGTTGG 70 OsSUS5 LOC_Os04g24430 GCATTCGGGCTAACGGTCAT/ATTCCTGCCTCCCTGTCGT 140 OsSUS7 LOC_Os04g17650 TACAGGCACCAGATCCTAC/CTGCTGCTTGATTCTTTGA 200 OsSPS1 LOC_Os01g69030 GGCACAGCAAGACACTCCC/CGCCACGAACTAGACCATG 134 OsSPS11 LOC_Os11g12810 CCAAGCACCACAAGCAGACCG/GGCCGAATGGCTCGACAAGA 99 OsNR1.2 LOC_Os08g36500 ACATCTTCGTGTGCGCCA/GGGGAACTTGGGGTGCTC 134 OsNR2 LOC_Os02g53130 GAGTTCCGGTACATCGGAGT/TGCACAACCATCCATCAATC 88 OsGSNOR1 LOC_Os02g57040 TACTGGCCTCGGAGCAGT/TTGCCCCCTCAGCAACTG 104 OsActin LOC_Os03g50885 GACCTTCAACACCCCTGCTA/ACAGTGTGGCTGACACCATC 114 1.5 数据处理
利用Microsoft Excel 2019进行数据整理,采用SPSS 25.0软件进行ANOVA差异性分析,使用GraphPad Prism 8、Origin Pro 2021和Microsoft Visio软件绘制图形。
2. 结果与分析
2.1 碱胁迫下不同浓度SNP对萌发期水稻生长指标的影响
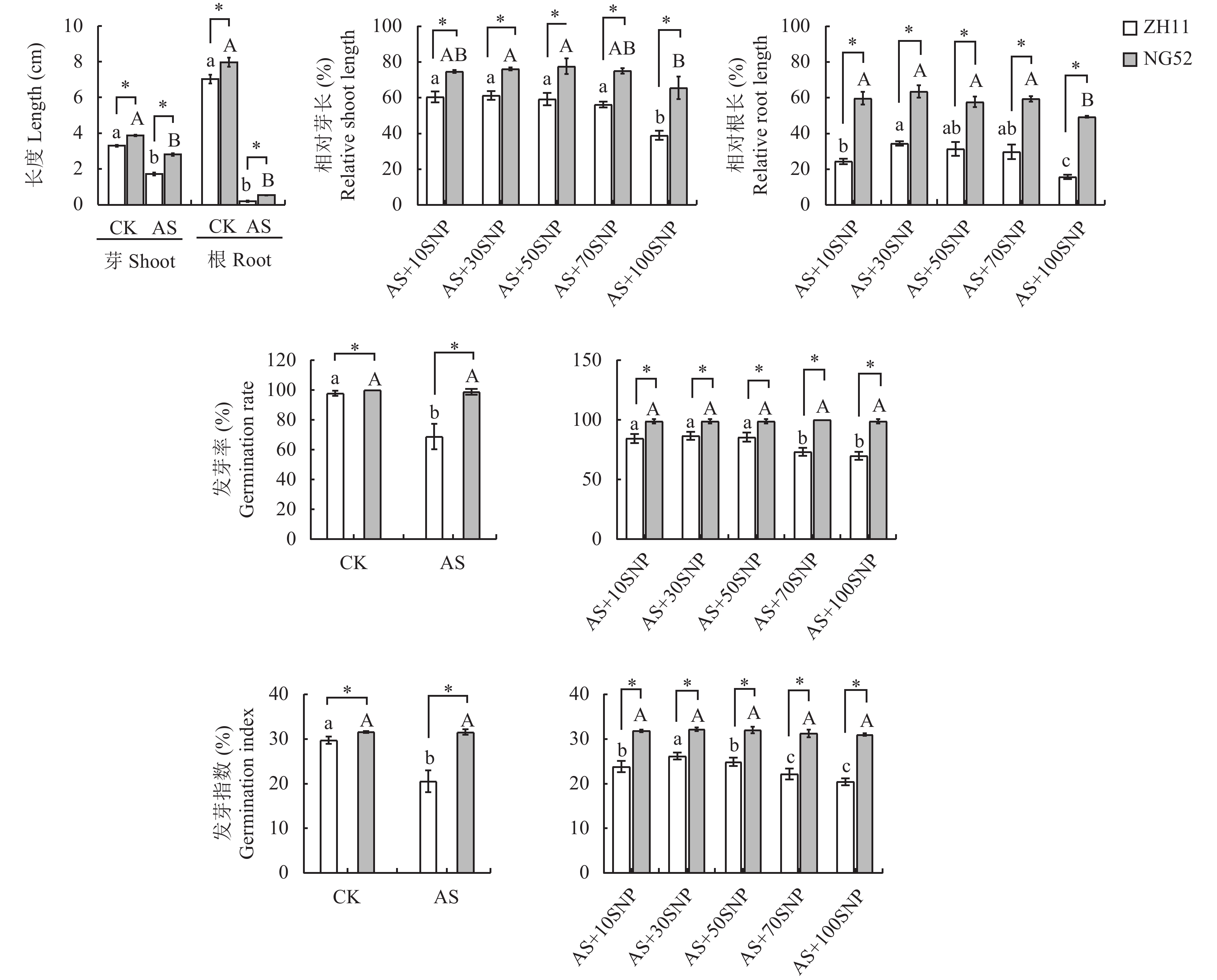
与正常条件(CK)相比,碱胁迫(AS)处理显著抑制了ZH11和NG52的芽长和根长(图1),AS+10~70 μmol/L SNP处理间2个品种的相对芽长和相对根长均无显著差异,而AS+100 μmol/L SNP处理显著抑制了它们的芽长和根长。相对于NG52,萌发期ZH11对碱胁迫更为敏感。统计结果进一步表明,AS+10~50 μmol/L SNP处理下ZH11种子萌发率显著高于AS+70~100 μmol/L SNP处理。其中,AS+30 μmol/L SNP处理下发芽指数显著高于其他浓度SNP处理。
![]() 图 1 不同浓度SNP处理对碱胁迫下萌发期水稻生长指标的影响注:CK—蒸馏水对照;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理 (20 mmol/L混合碱+X μmol/L 硝普钠,X = 10, 30, 50, 70, 100 μmol/L)。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *表示相同胁迫处理不同品种在0.05水平差异显著。Figure 1. Effects of different SNP concentrations on growth indexes of rice at germination stage under alkaline stressNote: CK—Distilled water control; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside (20 mmol/L mixed alkali + X μmol/L SNP, X = 10, 30, 50, 70, 100 μmol/L). Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. * indicates significant difference between two cultivars under the same stress treatment at the 0.05 level.
图 1 不同浓度SNP处理对碱胁迫下萌发期水稻生长指标的影响注:CK—蒸馏水对照;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理 (20 mmol/L混合碱+X μmol/L 硝普钠,X = 10, 30, 50, 70, 100 μmol/L)。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *表示相同胁迫处理不同品种在0.05水平差异显著。Figure 1. Effects of different SNP concentrations on growth indexes of rice at germination stage under alkaline stressNote: CK—Distilled water control; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside (20 mmol/L mixed alkali + X μmol/L SNP, X = 10, 30, 50, 70, 100 μmol/L). Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. * indicates significant difference between two cultivars under the same stress treatment at the 0.05 level.2.2 碱胁迫下SNP对萌发期水稻碳代谢的影响
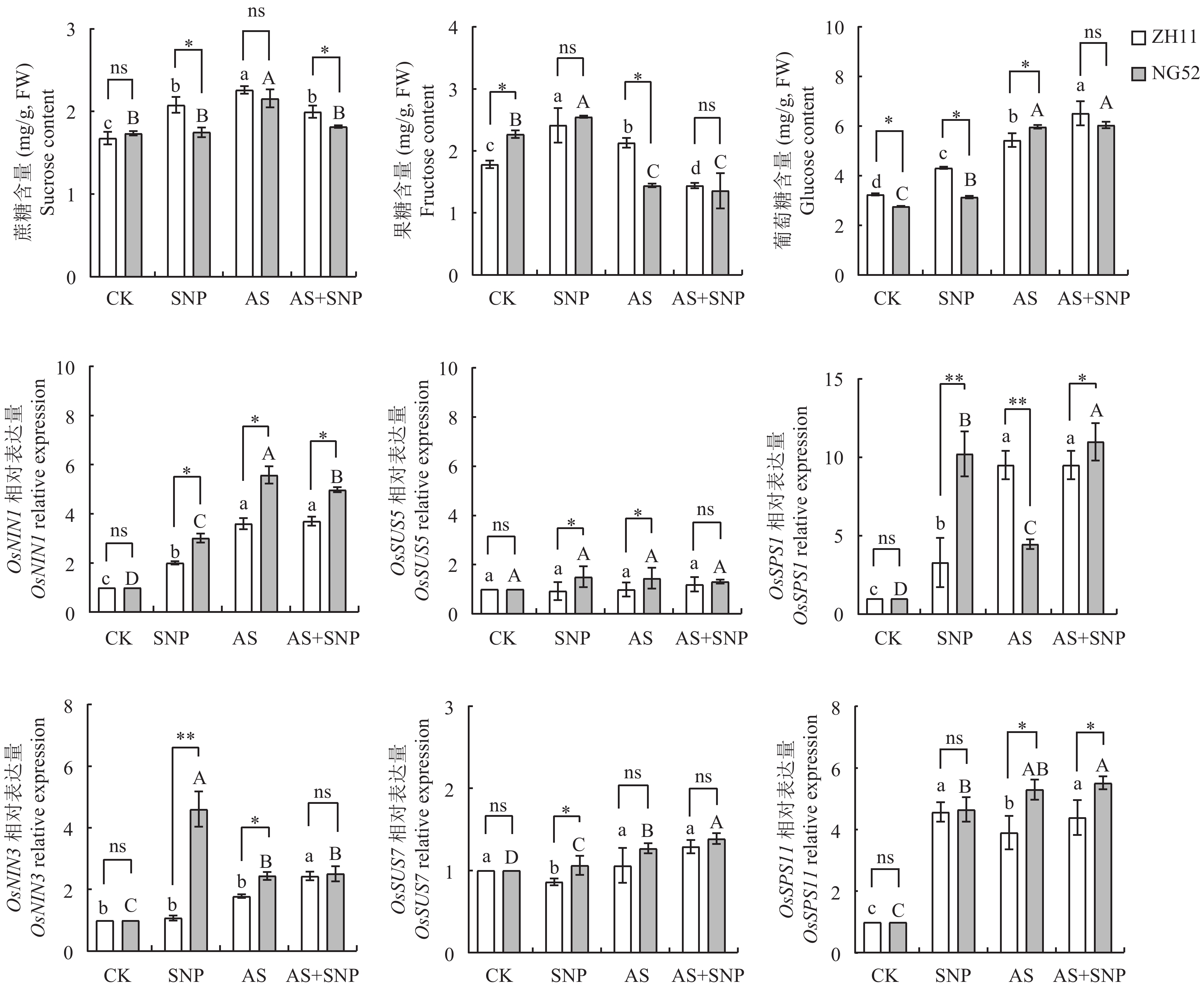
由图2可知,相比于CK处理,AS处理显著增加了ZH11和NG52中蔗糖、葡萄糖含量和上调了糖类合成酶基因OsNIN1、OsNIN3、OsSPS1、OsSPS11表达。与AS处理相比,AS+SNP处理显著降低了2个品种中蔗糖含量。其中,NG52中蔗糖含量降幅显著大于ZH11,降幅达到9.3% (图2),OsSPS1、OsSPS11基因表达量增幅显著大于ZH11,分别达到0.12、0.25倍,而果糖、葡萄糖含量和OsSUS5、OsSUS7、OsNIN3基因表达量在两个品种间无显著差异(P>0.05)。
![]() 图 2 碱胁迫下SNP对萌发期水稻碳代谢的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 2. Effects of SNP on carbon metabolism of rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 2 碱胁迫下SNP对萌发期水稻碳代谢的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 2. Effects of SNP on carbon metabolism of rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.2.3 碱胁迫下SNP对萌发期水稻氮代谢的影响
2.3.1 碱胁迫下SNP对萌发期水稻氮代谢合成途径的影响
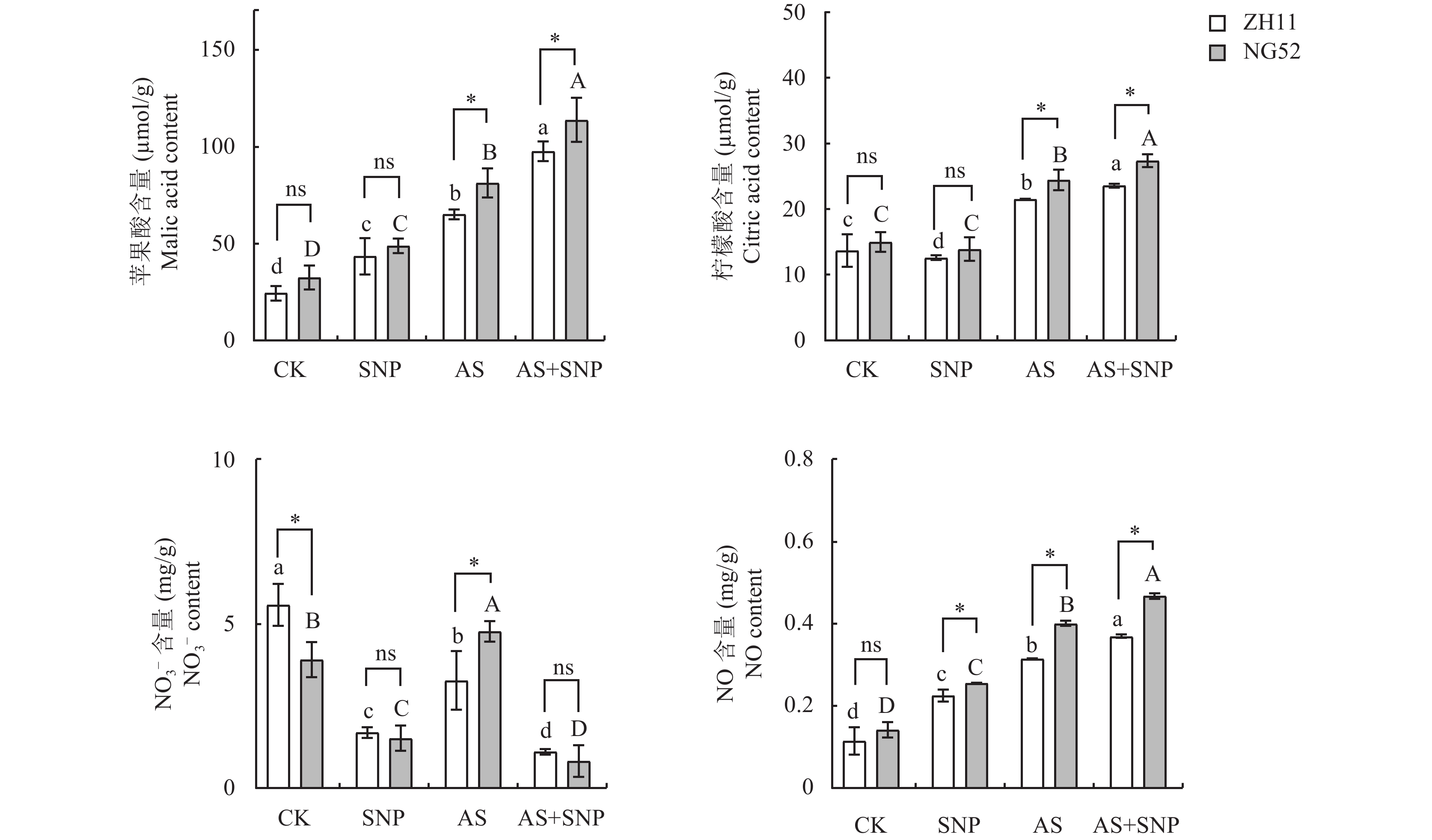
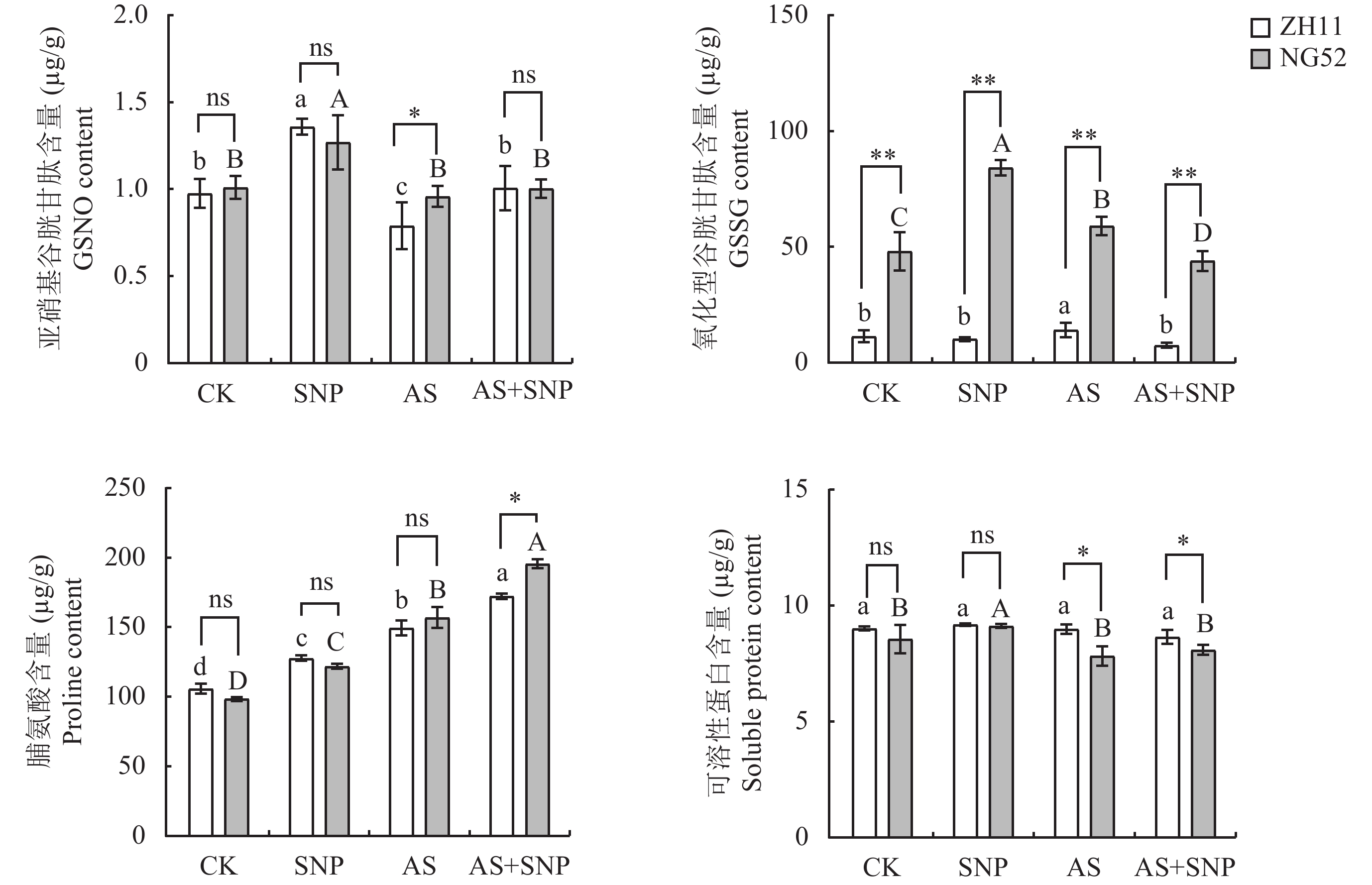
由图3可知,与CK相比,AS处理显著增加了ZH11和NG52中苹果酸、柠檬酸和一氧化氮含量。相比于AS处理,AS+SNP处理显著增加了两个品种中苹果酸、柠檬酸和NO含量,而显著降低了NO3−离子含量。AS+SNP处理下,NG52中苹果酸、柠檬酸和一氧化氮含量较ZH11显著增加,分别增加了20%、14.29%和21% (P<0.05)。
![]() 图 3 碱胁迫下SNP对萌发期水稻氮代谢合成的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 3. Effects of SNP on nitrogen metabolism and synthesis in rice at germination stage under alkaline stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 3 碱胁迫下SNP对萌发期水稻氮代谢合成的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 3. Effects of SNP on nitrogen metabolism and synthesis in rice at germination stage under alkaline stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.2.3.2 碱胁迫下SNP对萌发期水稻氮代谢合成关键酶活性及其基因表达量的影响
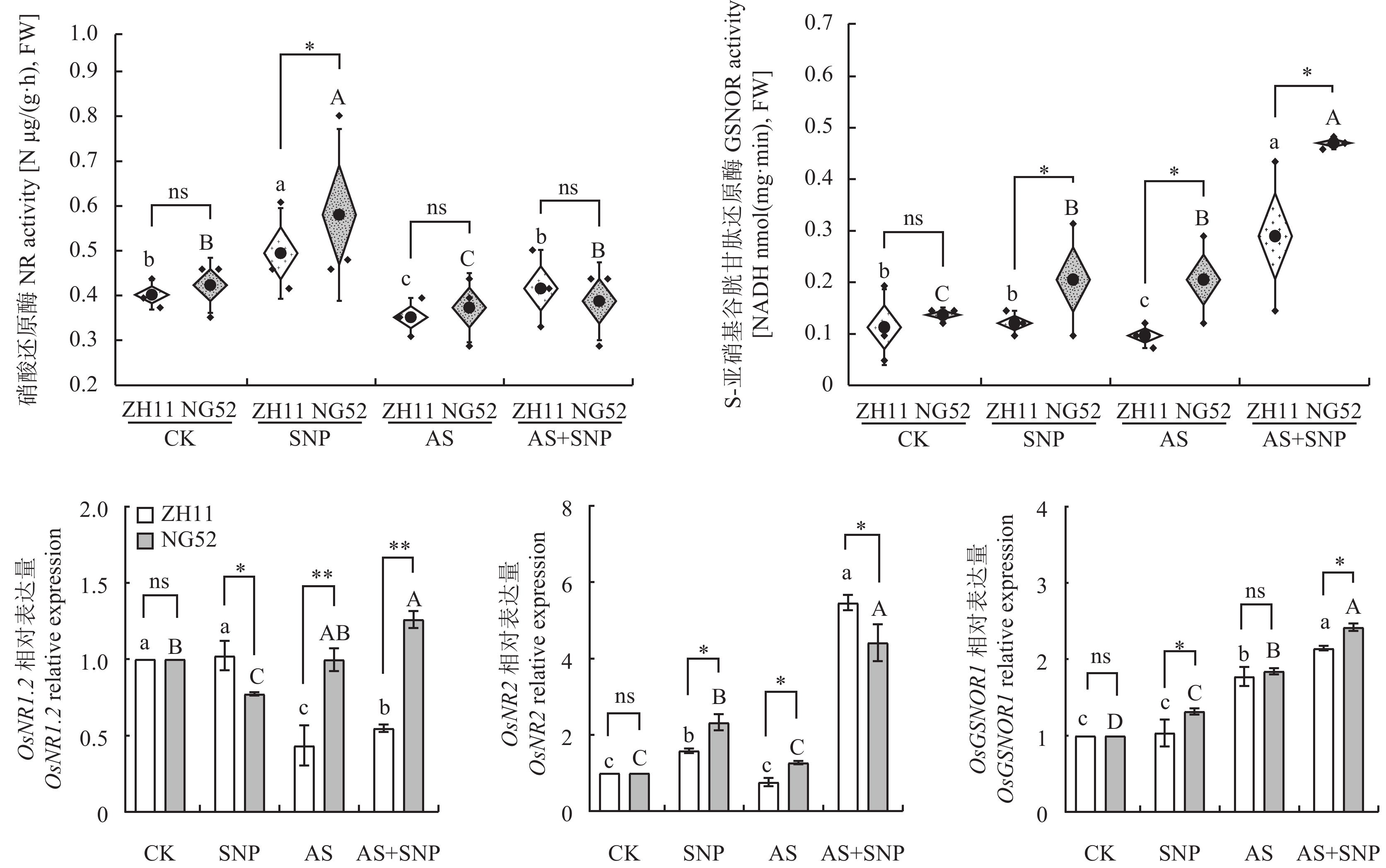
由图4可知,与CK相比,AS处理降低了ZH11和NG52中硝酸还原酶活性,而上调OsGSNOR1基因表达。相比于AS, AS+SNP处理增强了两个品种中硝酸还原酶、S-亚硝基谷胱甘肽还原酶活性,上调了OsNR2、OsGSNOR1基因表达。在AS+SNP处理下,相较于ZH11,NG52中S-亚硝基谷胱甘肽还原酶活性和OsNR1.2和OsGSNOR1基因表达量显著提高(P<0.05),分别增加了68%和1.32、0.15倍。
![]() 图 4 碱胁迫下SNP对萌发期水稻氮代谢合成关键酶活性及其基因表达量的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 4. Effects of SNP on key enzyme activity and gene expression of nitrogen metabolism and synthesis in rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 4 碱胁迫下SNP对萌发期水稻氮代谢合成关键酶活性及其基因表达量的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 4. Effects of SNP on key enzyme activity and gene expression of nitrogen metabolism and synthesis in rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.2.3.3 碱胁迫下SNP对萌发期水稻氮代谢分解途径的影响
由图5可知,与CK相比,AS处理显著增加了ZH11和NG52中氧化型谷胱甘肽和脯氨酸含量。相比于AS,AS+SNP处理显著降低了两个品种中氧化型谷胱甘肽含量,但却增加了脯氨酸含量。在AS+SNP处理下,NG52相较于ZH11,其氧化型谷胱甘肽和脯氨酸含量显著提高,分别增加了470.6%和11.2%,可溶性蛋白含量显著降低,降低了6.50%,而亚硝基谷胱甘肽含量在两个品种间无显著差异。
![]() 图 5 碱胁迫下SNP对萌发期水稻氮代谢分解途径的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 5. Effects of SNP on nitrogen metabolism and decomposition pathway of rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 5 碱胁迫下SNP对萌发期水稻氮代谢分解途径的影响注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。Figure 5. Effects of SNP on nitrogen metabolism and decomposition pathway of rice at germination stage under alkali stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.2.4 碱胁迫下SNP处理萌发期水稻碳氮代谢指标相关性和主成分分析
2.4.1 相关性分析
对不同处理下ZH11和NG52中22个碳、氮代谢相关指标进行相关性分析,结果表明葡萄糖含量与苹果酸、柠檬酸、脯氨酸和一氧化氮含量呈显著正相关,蔗糖合成酶基因(OsNIN1)与柠檬酸和一氧化氮含量呈显著正相关(图6),说明碳代谢途径中的碳水化合物一部分合成葡萄糖提供能量,一部分流入氮代谢合成有机酸,水稻中碳氮代谢联系紧密,共同促进水稻早期生长。
![]() 图 6 碱胁迫下SNP处理萌发期水稻碳氮代谢指标相关性分析注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。*、**分别表示指标之间在0.05、0.01水平相关显著。Figure 6. Correlation analysis of carbon and nitrogen metabolism indexes of rice at germination stage in SNP treatments under alkali stressNote: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—oxidized glutathione. * and ** indicate significant correlation among indexes at the 0.05 and 0.01 levels, respectively.
图 6 碱胁迫下SNP处理萌发期水稻碳氮代谢指标相关性分析注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。*、**分别表示指标之间在0.05、0.01水平相关显著。Figure 6. Correlation analysis of carbon and nitrogen metabolism indexes of rice at germination stage in SNP treatments under alkali stressNote: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—oxidized glutathione. * and ** indicate significant correlation among indexes at the 0.05 and 0.01 levels, respectively.2.4.2 主成分分析
进一步对不同处理下ZH11和NG52中22个碳、氮代谢相关指标进行主成分分析(图7)。按照特征值大于1的准则提取主成分,综合成3个主成分因子,其中第一主成分的贡献率较高。对ZH11来说,第一主成分中葡萄糖(0.298)、OsNIN3 (0.289)和NO (0.295)载荷绝对值较高,第二主成分中亚硝基谷胱甘肽(0.316)、氧化型谷胱甘肽(0.313)和硝酸还原酶(0.485)载荷绝对值较高,同理可得,第三主成分中果糖(0.452)、OsSPS11 (0.4260)和亚硝基谷胱甘肽(0.385)载荷绝对值较高。对NG52而言,第一主成分中葡萄糖(0.324)、柠檬酸(0.302)和NO (0.321)载荷绝对值较高,第二主成分中OsNIN3 (0.442)、亚硝基谷胱甘肽(0.363)和氧化型谷胱甘肽(0.364)载荷绝对值较高,同理可得,在第三主成分中蔗糖(0.500)、果糖(0.500)和脯氨酸(0.256)载荷绝对值较高。综上所述,两个品种中葡萄糖、果糖、一氧化氮、亚硝基谷胱甘肽和氧化型谷胱甘肽可作为碳、氮代谢的关键指标。
![]() 图 7 碱胁迫下SNP对萌发期水稻碳氮代谢指标主成分分析注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。箭头代表原始变量,其中方向代表原始变量与主成分的相关性,长度代表原始数据对主成分的贡献度,长度越长贡献度越大。Figure 7. Principal component analysis of SNP on carbon and nitrogen metabolism indexes of rice at germination stage under alkali stressNote: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione. The arrows represent the original variables, where the direction represents the correlation of the original variables with the principal components and the length represents the contribution of the original data to the principal components, the longer the length, the greater
图 7 碱胁迫下SNP对萌发期水稻碳氮代谢指标主成分分析注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。箭头代表原始变量,其中方向代表原始变量与主成分的相关性,长度代表原始数据对主成分的贡献度,长度越长贡献度越大。Figure 7. Principal component analysis of SNP on carbon and nitrogen metabolism indexes of rice at germination stage under alkali stressNote: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione. The arrows represent the original variables, where the direction represents the correlation of the original variables with the principal components and the length represents the contribution of the original data to the principal components, the longer the length, the greater3. 讨论
3.1 碱胁迫下外源SNP对萌发期水稻碳代谢的影响
碳代谢是指植物对碳源吸收、运输和转化,其包括将CO2同化为碳水化合物的碳同化过程,有机碳化物运输转化合成蔗糖后进一步转化为单糖的运输和转化过程,以及以蔗糖或淀粉形式储存的碳积累过程[20]。研究表明,水稻幼苗期叶面喷施血红素加氧酶1诱导剂,加速NaCl处理下淀粉向果糖和蔗糖的降解,提高了蔗糖和可溶性糖的浓度,同时增强了碳水化合物的代谢,缓解了盐胁迫引起的渗透胁迫,维持了植株的正常生长[21]。糖类化合物作为植物生长的必需物质,其代谢不仅影响蛋白质的组成和脂类的分解,还影响核酸的组成和代谢等[22]。糖类化合物的合成代谢在“源”器官中最旺盛,此时有利于源端光合产物向库端运输,从而提高“库”器官光合同化物的累积,糖类化合物含量的高低影响植物体内“源−库−源”协调状况,因而可直接反映植物的碳代谢[23−24]。例如,外源TRE (海藻糖)增强了植物糖类物质的积累,降低营养器官中淀粉含量,有助于淀粉在源―库间的转运和分配,因此源库关系与植物碳氮代谢密切相关[25]。目前,大多数研究集中在源库关系建立后的碳氮代谢的相互转化对作物生长的影响,而有关萌发期和生长早期作物体内碳氮代谢的变化需要深入研究。本试验结果表明,相比于CK处理,在AS处理下ZH11和NG52蔗糖含量均增加,说明水稻幼芽响应碱胁迫伤害,开始大量合成蔗糖,同时蔗糖合成相关基因OsNIN1上调表达,促进蔗糖积累,增强碳水化合物代谢。相比于AS处理,在AS+SNP处理下ZH11中蔗糖、果糖含量降低,葡萄糖含量增加,且OsNIN1、OsSPS11合成基因表达量上调。而NG52蔗糖含量降低,果糖和葡萄糖含量无显著变化。可能的原因是,在AS+SNP处理下,外源SNP加速碳代谢,NG52作为耐碱性材料碳代谢能力更强,受到碱胁迫刺激后蔗糖大量合成,但同时蔗糖也快速分解转化为其他中间产物抵御胁迫伤害,即蔗糖向果糖、葡萄糖小分子物质转化,也同时向氨基酸物质合成方向进行,但向氨基酸合成转化速率大于向果糖、葡萄糖的转化速率,从而NG52蔗糖含量降低,葡萄糖和果糖含量无显著差异,有机酸显著积累。上述研究结果说明胁迫会对碳代谢产生影响,外源物添加会促进碳水化合物的代谢,增强碳代谢,缓解碱胁迫。
3.2 碱胁迫下外源SNP对萌发期水稻氮代谢的影响
植物碳代谢的核心是碳水化合物的合成及相互转化,其中非结构性碳水化合物(可溶性糖等)是作物光合和生长的主要中间贮藏产物[26]。碳水化合物的形成、转化及蛋白质的合成与分解均反映碳代谢变化,植物氨基酸和蛋白质的合成反映氮代谢变化,氮代谢通过氮同化作用吸收氮素,供给植物生长发育[27]。将碳、氮代谢密切相连的物质之一是植物细胞中的有机酸,有机酸既是碳代谢主要中间产物又与氮代谢密切相关,有机酸代谢的中间产物,如草酰乙酸和α-酮戊二酸,为转氨和氨基酸合成提供碳骨架进而参与氮代谢[28−29]。Chen等[28]研究结果表明,随着氮供应水平的降低,水稻叶片和根系中硝酸还原酶、谷氨酰胺合成酶和谷氨酸合成酶等N代谢相关酶的活性降低,总可溶性蛋白含量降低,最终导致植物生长受到抑制。缺氮的信号可能不直接作用于三羧酸循环 (tricarboxylic acid cycle,TCA),而是作用于细胞质中有机酸生物合成步骤[28,30]。师瑞红等[31]研究发现,1.5 mmol/L铝胁迫下水稻幼苗根系受到轻微伤害,加入有机酸后却提高了胁迫下根系中活性氧清除酶的活性,缓解了发芽期水稻幼苗的Al毒伤害。本研究结果也表明,在碱胁迫下ZH11和NG52材料中苹果酸、柠檬酸含量均增加;碱胁迫下施加外源SNP处理ZH11和NG52中苹果酸、柠檬酸含量均高于AS处理,且NG52材料中有机酸含量显著高于ZH11 (图3),说明外源SNP添加诱导有机酸进一步累积,与师瑞红等[31]的研究一致。进一步说明NG52作为耐碱材料,有机酸积累程度更高,氮代谢能力更强,对碱胁迫缓解效应也更加显著。
硝酸盐是植物利用无机氮的重要来源,其中NO3−是植物在通气良好土壤中吸收氮源的主要形式,植物将吸收的无机氮化物转化为可利用的有机氮化物,将从外界吸收的NO3−合成氨基酸,后通过一系列转化合成过程形成各种蛋白质底物,供给植物生长发育[32−33]。有机氮化物如脯氨酸作为额外氮元素的储存形式,其合成被认为是同化多余氨的一种方式,其积累有利于植物在逆境环境中抵御外界伤害[34]。大量氨累积会导致氨毒害,植物体内的氨必须立即同化为有机氮以消除其对细胞的毒性,促进氮代谢恢复正常[35]。消除氨毒害的过程需先经过一个硝酸还原过程,此过程需要硝酸还原酶的参与[36−37]。同时为保护机体免受亚硝化胁迫,S-亚硝基谷胱甘肽还原酶通过亚硝基谷胱甘肽途径调节细胞内NO含量[38−39]。本研究结果表明,相比于AS处理,在AS+SNP处理下ZH11和NG52中脯氨酸含量均增加。扈雪欢等[40]研究结果表明,SA通过提升盐胁迫下颠茄对无机氮的吸收,并通过诱导谷氨酰胺合成酶、谷氨酸合成酶活性增加,加强对铵态氮的同化,以缓解因氮源不足造成的伤害。本研究结果与扈雪欢等[40]研究结果一致,本研究表明相比于AS处理,在AS+SNP处理下ZH11和NG52中NO3−离子含量降低,NO含量增加。本试验研究结果也表明,AS+SNP处理相比于AS处理,ZH11和NG52两材料硝酸还原酶、S-亚硝基谷胱甘肽还原酶活性显著增加,氧化型谷胱甘肽含量显著降低,相关合成基因OsNR2、OsGSNOR1相对表达量也显著上调。综上,说明外施适宜浓度的SNP能有效缓解碱胁迫对水稻幼芽氮代谢功能的抑制作用,增强氮代谢能力,从而提高抗碱性。
3.3 碱胁迫下外源SNP对萌发期水稻碳氮代谢的调控机制分析
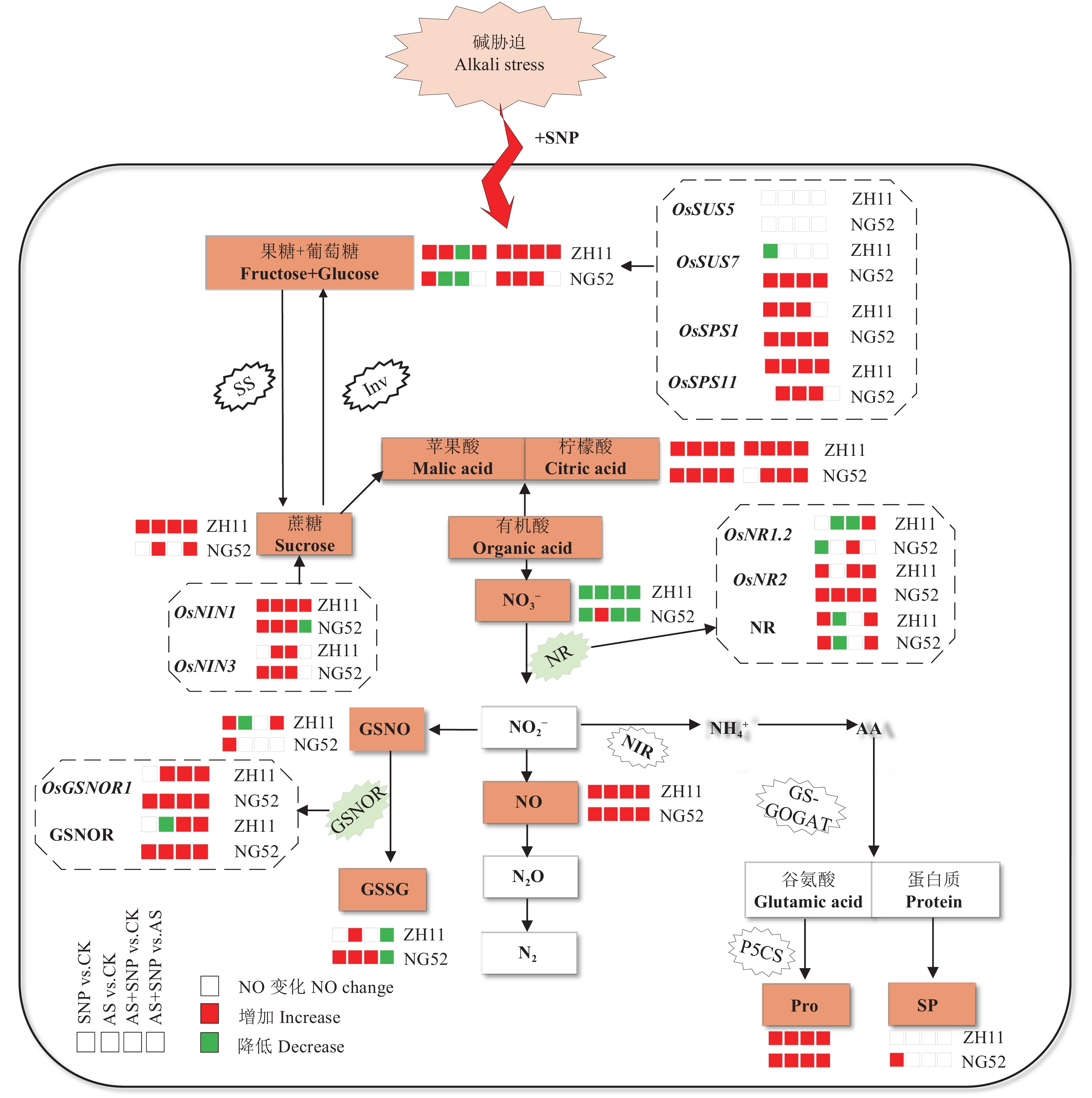
碳、氮代谢是植物体内两大基本的生理代谢过程,对植物的生长发育具有极其重要的作用[41]。在生长前期主要以氮代谢为主,生长发育中期碳代谢和氮代谢并重,生长后期以碳代谢为主,碳氮代谢平衡决定着植物体营养生长和生殖生长的关系[42]。植株通过碳氮平衡调节不同代谢过程以适应生长需求,这些过程需要考虑碳氮吸收和同化,干物质的运输、分配和储存,及呼吸消耗等问题[42−43]。徐冉等[44]研究表明,氮素在调节植株氮代谢的同时也在间接影响水稻碳代谢,进而共同影响水稻产量。中后期施用氮肥能够促进根系的生长发育,维持根系活力。陈心怡等[11]研究也发现,光强和氮肥互作对结实期水稻碳氮代谢产生影响,在提升水稻氮代谢的同时又能够进一步提高水稻叶片的净光合速率,增强碳代谢能力,促进籽粒中碳氮代谢产物的积累,进而对水稻产量和品质产生影响。这两种代谢活动需要共同的还原力以及碳骨架,碳代谢和氮代谢相互关联。本研究表明,碱胁迫下施加外源SNP处理增加了碳水化合物(蔗糖、果糖、葡萄糖)合成与转化,促进了碳氮代谢中间产物有机酸(苹果酸、柠檬酸)的积累。同时增强了硝酸还原酶和S-亚硝基谷胱甘肽还原酶活性,降低NO3−含量,有助于无机氮被同化为氨基酸,进而合成了有机氮化合物(脯氨酸、可溶性蛋白),表明添加外源SNP调控了碱胁迫下水稻的碳氮代谢水平,维持了碱胁迫下萌发期水稻的生长(图8)。
![]() 图 8 碱胁迫下SNP处理萌发期水稻碳氮调控机制模式图注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。SP—可溶性蛋白;Pro—脯氨酸;SS—蔗糖合成酶;Inv—转化酶;NR—硝酸还原酶;NIR—亚硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GS-GOGAT—谷氨酰胺/谷氨酸合成酶;P5CS—吡咯啉-5-羧酸合成酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽;AA—氨基酸。Figure 8. The regulation model diagram mechanism SNP on carbon and nitrogen in rice at germination stage under alkaline stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. SP—Soluble protein; Pro—Proline; SS—Sucrose synthase; Inv—Invertase; NR—Nitrate reductase; NIR—Nitrite reductase; GSNOR—S-nitroso glutathione reductase; GS-GOGAT—Glutamine/ glutamate synthase; P5CS—Pyrroline-5-carboxylic acid synthase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione; AA—amino acid.
图 8 碱胁迫下SNP处理萌发期水稻碳氮调控机制模式图注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。SP—可溶性蛋白;Pro—脯氨酸;SS—蔗糖合成酶;Inv—转化酶;NR—硝酸还原酶;NIR—亚硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GS-GOGAT—谷氨酰胺/谷氨酸合成酶;P5CS—吡咯啉-5-羧酸合成酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽;AA—氨基酸。Figure 8. The regulation model diagram mechanism SNP on carbon and nitrogen in rice at germination stage under alkaline stressNote: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. SP—Soluble protein; Pro—Proline; SS—Sucrose synthase; Inv—Invertase; NR—Nitrate reductase; NIR—Nitrite reductase; GSNOR—S-nitroso glutathione reductase; GS-GOGAT—Glutamine/ glutamate synthase; P5CS—Pyrroline-5-carboxylic acid synthase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione; AA—amino acid.4. 结论
施加外源SNP调控了碱胁迫下水稻的碳氮代谢,提升了碳氮代谢能力,改善了碱胁迫下水稻的萌发和早期生长。碱胁迫下,喷施外源SNP增加了ZH11和NG52两个水稻品种中苹果酸、柠檬酸、一氧化氮、脯氨酸含量和S-亚硝基谷胱甘肽还原酶活性,降低了蔗糖、NO3−含量,上调了氮代谢合成酶基因OsNR2、OsGSNOR1的表达。与碱胁迫敏感型品种ZH11相比,碱胁迫抗性品种NG52的蔗糖、果糖、葡萄糖、NO3−含量降低,苹果酸、柠檬酸、一氧化氮、氧化型谷胱甘肽、脯氨酸含量增加,硝酸还原酶、S-亚硝基谷胱甘肽还原酶活性增强,碳、氮代谢合成酶基因(OsNIN1、OsSUS5、OsSPS7、OsNR1.2、OsGSNOR1)表达量升高,反映出NG52的碳氮代谢能力强于ZH11。
-
图 1 不同浓度SNP处理对碱胁迫下萌发期水稻生长指标的影响
注:CK—蒸馏水对照;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理 (20 mmol/L混合碱+X μmol/L 硝普钠,X = 10, 30, 50, 70, 100 μmol/L)。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *表示相同胁迫处理不同品种在0.05水平差异显著。
Figure 1. Effects of different SNP concentrations on growth indexes of rice at germination stage under alkaline stress
Note: CK—Distilled water control; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside (20 mmol/L mixed alkali + X μmol/L SNP, X = 10, 30, 50, 70, 100 μmol/L). Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. * indicates significant difference between two cultivars under the same stress treatment at the 0.05 level.
图 2 碱胁迫下SNP对萌发期水稻碳代谢的影响
注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。
Figure 2. Effects of SNP on carbon metabolism of rice at germination stage under alkali stress
Note: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 3 碱胁迫下SNP对萌发期水稻氮代谢合成的影响
注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。
Figure 3. Effects of SNP on nitrogen metabolism and synthesis in rice at germination stage under alkaline stress
Note: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 4 碱胁迫下SNP对萌发期水稻氮代谢合成关键酶活性及其基因表达量的影响
注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。
Figure 4. Effects of SNP on key enzyme activity and gene expression of nitrogen metabolism and synthesis in rice at germination stage under alkali stress
Note: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 5 碱胁迫下SNP对萌发期水稻氮代谢分解途径的影响
注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。柱上不同大、小写字母分别表示宁梗52、中花11不同处理间在0.05水平差异显著。 *、**分别表示相同胁迫处理不同品种在0.05、0.01水平差异显著;ns表示差异不显著。
Figure 5. Effects of SNP on nitrogen metabolism and decomposition pathway of rice at germination stage under alkali stress
Note: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. Different capital and lowercase letters above the bars indicate significant difference among treatments of NG52 and ZH11 at the 0.05 level, respectively. *, ** indicate significant difference between two cultivars under the same stress treatment at the 0.05 and 0.01 levels, respectively; ns indicates no significant difference.
图 6 碱胁迫下SNP处理萌发期水稻碳氮代谢指标相关性分析
注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。*、**分别表示指标之间在0.05、0.01水平相关显著。
Figure 6. Correlation analysis of carbon and nitrogen metabolism indexes of rice at germination stage in SNP treatments under alkali stress
Note: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—oxidized glutathione. * and ** indicate significant correlation among indexes at the 0.05 and 0.01 levels, respectively.
图 7 碱胁迫下SNP对萌发期水稻碳氮代谢指标主成分分析
注:SP—可溶性蛋白;Pro—脯氨酸;NR—硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽。箭头代表原始变量,其中方向代表原始变量与主成分的相关性,长度代表原始数据对主成分的贡献度,长度越长贡献度越大。
Figure 7. Principal component analysis of SNP on carbon and nitrogen metabolism indexes of rice at germination stage under alkali stress
Note: SP—Soluble protein; Pro—Proline; NR—Nitrate reductase; GSNOR—S-nitroso glutathione reductase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione. The arrows represent the original variables, where the direction represents the correlation of the original variables with the principal components and the length represents the contribution of the original data to the principal components, the longer the length, the greater
图 8 碱胁迫下SNP处理萌发期水稻碳氮调控机制模式图
注:CK—蒸馏水对照;SNP—硝普钠处理;AS—碱胁迫处理 (20 mmol/L混合碱处理);AS+SNP—碱胁迫+硝普钠处理。SP—可溶性蛋白;Pro—脯氨酸;SS—蔗糖合成酶;Inv—转化酶;NR—硝酸还原酶;NIR—亚硝酸还原酶;GSNOR—S-亚硝基谷胱甘肽还原酶;GS-GOGAT—谷氨酰胺/谷氨酸合成酶;P5CS—吡咯啉-5-羧酸合成酶;GSNO—S-亚基谷胱甘肽;GSSG—氧化型谷胱甘肽;AA—氨基酸。
Figure 8. The regulation model diagram mechanism SNP on carbon and nitrogen in rice at germination stage under alkaline stress
Note: CK—Distilled water control; SNP—Sodium nitroprusside; AS—Alkali stress (20 mmol/L mixed alkali treatment); AS + SNP—Alkali stress + sodium nitroprusside. SP—Soluble protein; Pro—Proline; SS—Sucrose synthase; Inv—Invertase; NR—Nitrate reductase; NIR—Nitrite reductase; GSNOR—S-nitroso glutathione reductase; GS-GOGAT—Glutamine/ glutamate synthase; P5CS—Pyrroline-5-carboxylic acid synthase; GSNO—S-subunit glutathione; GSSG—Oxidized glutathione; AA—amino acid.
表 1 检测的基因和RT-qPCR引物序列
Table 1 Genes examined and RT-qPCR primer sequences
基因名称
Gene symbol登录号
RGAP ID引物序列 (5′—3′) (正向/反向)
Primer sequence (5′—3′) (forward/reverse)产物大小 (bp)
Product sizeOsNIN1 LOC_Os03g20020 CTATTCTGCTTTGCGTTGT/CTGAGCCTGTAGTTGATGG 87 OsNIN3 LOC_Os02g32730 TGCGGGAGTCCGTGGTTTA/GCATCATTCGGGTCGTTGG 70 OsSUS5 LOC_Os04g24430 GCATTCGGGCTAACGGTCAT/ATTCCTGCCTCCCTGTCGT 140 OsSUS7 LOC_Os04g17650 TACAGGCACCAGATCCTAC/CTGCTGCTTGATTCTTTGA 200 OsSPS1 LOC_Os01g69030 GGCACAGCAAGACACTCCC/CGCCACGAACTAGACCATG 134 OsSPS11 LOC_Os11g12810 CCAAGCACCACAAGCAGACCG/GGCCGAATGGCTCGACAAGA 99 OsNR1.2 LOC_Os08g36500 ACATCTTCGTGTGCGCCA/GGGGAACTTGGGGTGCTC 134 OsNR2 LOC_Os02g53130 GAGTTCCGGTACATCGGAGT/TGCACAACCATCCATCAATC 88 OsGSNOR1 LOC_Os02g57040 TACTGGCCTCGGAGCAGT/TTGCCCCCTCAGCAACTG 104 OsActin LOC_Os03g50885 GACCTTCAACACCCCTGCTA/ACAGTGTGGCTGACACCATC 114 -
[1] Liu L L, Wang B S. Protection of halophytes and their uses for cultivation of saline-alkali soil in China[J]. Biology, 2021, 10(5): 353. DOI: 10.3390/biology10050353
[2] Flowers T J, Colmer T D. Salinity tolerance in halophytes[J]. New Phytologist, 2008, 179(4): 945−963. DOI: 10.1111/j.1469-8137.2008.02531.x
[3] Penfield S. Seed dormancy and germination[J]. Current Biology, 2017, 27(17): R874−R878. DOI: 10.1016/j.cub.2017.05.050
[4] 金梦野, 李小华, 李昉泽, 黄占斌. 盐碱复合胁迫对水稻种子发芽的影响[J]. 中国生态农业学报, 2020, 28(4): 566−574. Jin M Y, Li X H, Li F Z, Huang Z B. Effects of mixed saline-alkali stress on germination of rice[J]. Chinese Journal of Eco-Agriculture, 2020, 28(4): 566−574.
[5] Ling T F, Xuan W, Fan Y R, et al. The effect of exogenous glucose, fructose and NO donor sodium nitroprusside (SNP) on rice seed germination under salt stress[J]. Journal of Plant Physiology and Molecular Biology, 2005, 31(2): 205−212.
[6] Huang J, Zhu C Q, Hussain S, et al. Effects of nitric oxide on nitrogen metabolism and the salt resistance of rice (Oryza sativa L.) seedlings with different salt tolerances[J]. Plant Physiology and Biochemistry, 2020, 155: 374−383. DOI: 10.1016/j.plaphy.2020.06.013
[7] Ren M F, Liu S Z, Mao G L, et al. Simultaneous application of red and blue light regulate carbon and nitrogen metabolism, induces antioxidant defense system and promote growth in rice seedlings under low light stress[J]. International Journal of Molecular Sciences, 2023, 24(13): 10706. DOI: 10.3390/ijms241310706
[8] Ma X Z, Yu X X, Cui G C, et al. Methyl jasmonate mitigates osmotic stress by regulating carbon and nitrogen metabolism of glycyrrhiza uralensis seedlings subjected to salt stress[J]. Acta Physiologiae Plantarum, 2023, 45(8): 96. DOI: 10.1007/s11738-023-03574-z
[9] Baslam M, Mitsui T, Sueyoshi K, Ohyama T. Recent advances in carbon and nitrogen metabolism in C3 plants[J]. International Journal of Molecular Sciences, 2020, 22(1): 318. DOI: 10.3390/ijms22010318
[10] Liang Y P, Wang J Y, Zeng F L, et al. Photorespiration regulates carbon nitrogen metabolism by magnesium chelatase D subunit in rice[J]. Journal of Agricultural and Food Chemistry., 2021, 69(1): 112−125. DOI: 10.1021/acs.jafc.0c05809
[11] 陈心怡, 朱盈, 马中涛, 等. 光强和氮肥互作对南方软米粳稻灌浆结实期碳氮代谢影响及其与产量品质间关系[J]. 作物学报, 2023, 49(11): 3042−3062. Chen X Y, Zhu Y, Ma Z T, et al. Effects of light intensity and nitrogen fertilizer interaction on carbon and nitrogen metabolism at grain-filling stage and its relationship with yield and quality of southern soft japonica rice[J]. Acta Agronomica Sinica, 2023, 49(11): 3042−3062.
[12] 吴旭红, 罗贵强, 冯晶. 外源一氧化氮对Na2CO3胁迫下南瓜幼苗碳氮代谢的影响[J]. 干旱地区农业研究, 2017, 35(3): 151−158. DOI: 10.7606/j.issn.1000-7601.2017.03.24 Wu X H, Luo G Q, Feng J. Effects of exogenous nitric oxide on the growth and carbon and nitrogen metabolism of pumpkin seedlings under Na2CO3 stress[J]. Agricultural Research in the Arid Areas, 2017, 35(3): 151−158. DOI: 10.7606/j.issn.1000-7601.2017.03.24
[13] 石亚飞, 闵炜芳, 摆小蓉, 等. 外源物调节碱胁迫水稻生理特性及相关基因表达的效应[J]. 植物营养与肥料学报, 2023, 29(5): 813−825. DOI: 10.11674/zwyf.2022582 Shi Y F, Min W F, Bai X R, et al. Regulatory effects of different exogenous substances on physiological characteristics and gene expression of rice seedlings under alkali stress[J]. Journal of Plant Nutrition and Fertilizers, 2023, 29(5): 813−825. DOI: 10.11674/zwyf.2022582
[14] 路旭平, 李芳兰, 石亚飞, 等. 不同水稻品种幼苗响应碱胁迫的生理差异及胁迫等级构建[J]. 生态环境学报, 2021, 30(8): 1757−1768. Lu X P, Li F L, Shi Y F, et al. Physiological differences of seedlings of different rice varieties in response to alkali stress and construction of stress level[J]. Ecology and Environmental Sciences, 2021, 30(8): 1757−1768.
[15] 路旭平, 李芳兰, 马晓娟, 等. 不同碱敏感水稻品种根系对碱胁迫的生理响应策略[J]. 中国生态农业学报(中英文), 2021, 29(7): 1171−1184. Lu X P, Li F L, Ma X J, et al. Physiological response strategies of roots of different alkali-tolerant rice varieties to alkali stress[J]. Chinese Journal of Eco-Agriculture, 2021, 29(7): 1171−1184.
[16] 张娟伟, 石亚飞, 路旭平, 等. 种子萌发期粳稻种质资源耐旱性综合评价[J]. 核农学报, 2022, 36(11): 2093−2103. DOI: 10.11869/j.issn.100-8551.2022.11.2093 Zhang J W, Shi Y F, Lu X P, et al. Comprehensive evaluation of drought tolerance of japonica rice germplasm resources at seed germination stage[J]. Journal of Nuclear Agricultural Sciences, 2022, 36(11): 2093−2103. DOI: 10.11869/j.issn.100-8551.2022.11.2093
[17] 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000. Li H S. Plant physiological and biochemical experiment principle and technology[M]. Beijing : Higher Education Press, 2000.
[18] Hurali D T, Bhurta R, Tyagi S, et al. Analysis of NIA and GSNOR family genes and nitric oxide homeostasis in response to wheat-leaf rust interaction[J]. Scientific Reports, 2022, 12(1): 803. DOI: 10.1038/s41598-021-04696-5
[19] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method[J]. Methods, 2001, 25(4): 402−408. DOI: 10.1006/meth.2001.1262
[20] Ran F, Yuan Y J, Bai X M, et al. Carbon and nitrogen metabolism affects kentucky bluegrass rhizome expansion[J]. BMC Plant Biology, 2023, 23(1): 221. DOI: 10.1186/s12870-023-04230-x
[21] Meng F Y, Feng N J, Zheng D F, et al. Exogenous hemin alleviates NaCl stress by promoting photosynthesis and carbon metabolism in rice seedlings[J]. Scientific Reports, 2023, 13(1): 3497. DOI: 10.1038/s41598-023-30619-7
[22] Dwivedi A K, Singh V, Anwar K, et al. Integrated transcriptome, proteome and metabolome analyses revealed secondary metabolites and auxiliary carbohydrate metabolism augmenting drought tolerance in rice[J]. Plant Physiology and Biochemistry, 2023, 201: 107849. DOI: 10.1016/j.plaphy.2023.107849
[23] Griffiths C A, Paul M J, Foyer C H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions[J]. Biochim Biophys Acta, 2016, 1857(10): 1715−1725. DOI: 10.1016/j.bbabio.2016.07.007
[24] Gesch R W, Vu J C V, Boote K J, et al. Sucrose-phosphate synthase activity in mature rice leaves following changes in growth CO2 is unrelated to sucrose pool size[J]. New Phytologist, 2002, 154(1): 77−84. DOI: 10.1046/j.1469-8137.2002.00348.x
[25] 崔国际, 王传伟, 贺威, 等. 外源海藻糖对灌浆期高温胁迫下小麦灌浆特性和糖组分含量的影响[J]. 应用生态学报, 2023, 34(11): 3021−3029. Cui G J, Wang C W, He W, et al. Effects of exogenous trehalose on filling characteristic and sugar component content of wheat under high temperature stress during the filling period[J]. Chinese Journal of Applied Ecology, 2023, 34(11): 3021−3029.
[26] Chen X, Ji Y, Zhao W, et al. Fructose-6-phosphate-2-kinase/fructose-2, 6-bisphosphatase regulates energy metabolism and synthesis of storage products in developing rice endosperm[J]. Plant Science, 2023, 326: 111503. DOI: 10.1016/j.plantsci.2022.111503
[27] 任毛飞, 毛桂玲, 刘善振, 等. 光质对植物生长发育、光合作用和碳氮代谢影响的研究进展[J]. 植物生理学报, 2023, 59(7): 1211−1228. Ren M F, Mao G L, Liu S Z, et al. Research progress on the effects of light quality on plant growth and development, photosynthesis and carbon and nitrogen metabolism[J]. Plant Physiology Journal, 2023, 59(7): 1211−1228 .
[28] Chen L H, Cheng Z X, Xu M, et al. Effects of nitrogen deficiency on the metabolism of organic acids and amino acids in Oryza sativa[J]. Plants-Basel, 2022, 11(19): 2576. DOI: 10.3390/plants11192576
[29] Ribeiro B, Andrade P B, Baptista P, et al. Leucopaxillus giganteus mycelium: Effect of nitrogen source on organic acids and alkaloids[J]. Journal of Agricultural and Food Chemistry, 2008, 56(12): 4769−4774. DOI: 10.1021/jf8001526
[30] Famiani F, Bonghi C, Chen Z H, et al. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds-a review[J]. Front Plant Science, 2020, 11: 572601. DOI: 10.3389/fpls.2020.572601
[31] 师瑞红, 谢国生, 曾汉来, 张端品. 外源有机酸缓解水稻幼苗根系铝毒的生理机制[J]. 中国生态农业学报, 2007, 15(4): 97−101. Shi R H, Xie G S, Zeng H L, Zhang D P. Physiological mechanism of alleviating aluminum toxicity in rice seedling root by exogenous organic acids[J]. Chinese Journal of Eco-Agriculture, 2007, 15(4): 97−101.
[32] Lindstroem K, Mousavi S A. Effectiveness of nitrogen fixation in rhizobia[J]. Microbial Biotechnology, 2020, 13(5): 1314−1335. DOI: 10.1111/1751-7915.13517
[33] Dechorgnat J, Nguyen C T, Armengaud P, et al. From the soil to the seeds: The long journey of nitrate in plants[J]. Journal of Experimental Botany, 2011, 62(4): 1349−1359. DOI: 10.1093/jxb/erq409
[34] Brugiere N, Dubois F, Limami A M, et al. Glutamine synthetase in the phloem plays a major role in controlling proline production[J]. Plant Cell, 1999, 11(10): 1995−2012. DOI: 10.1105/tpc.11.10.1995
[35] Wickert E, Marcondes J, Lemos M V, Lemos E G M. Nitrogen assimilation in Citrus based on CitEST data mining[J]. Genetics and Molecular Biology, 2007, 30(3): 810−818.
[36] Pan Q N, Geng C C, Li D D, et al. Nitrate reductase-mediated ntric oxide regulates the leaf shape in arabidopsis by mediating the homeostasis of reactive oxygen species[J]. International Journal of Molecular Sciences, 2019, 20(9): 2235. DOI: 10.3390/ijms20092235
[37] Li H, Fu S H, Zhu J W, et al. Nitric oxide generated by piriformospora indica-induced nitrate reductase promotes tobacco growth by regulating root architecture and ammonium and nitrate transporter gene expression[J]. Journal of Plant Interactions, 2022, 17(1): 861−872. DOI: 10.1080/17429145.2022.2108926
[38] Zhang L, Song H Y, Li B H, et al. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice[J]. Journal of Experimental Botany, 2021, 72(12): 4548−4564. DOI: 10.1093/jxb/erab140
[39] Mostofa M G, Seraj Z I, Fujita M. Interactive effects of nitric oxide and glutathione in mitigating copper toxicity of rice (Oryza sativa L.) seedlings[J]. Plant Signaling & Behavior, 2015, 10(3): e991570.
[40] 扈雪欢, 宁欢欢, 刘光照, 吴能表. 外源SA对盐胁迫下颠茄生理生化、氮代谢及次生代谢的影响[J]. 草业学报, 2017, 26(11): 147−156. DOI: 10.11686/cyxb2017043 Hu X H, Ning H H, Liu G Z, Wu N B. Effects of exogenous SA on the physiological biochemical, nitrogen metabolism and secondary metabolism of atropa belladonna under NaCl stress[J]. Acta Prataculturae Sinica, 2017, 26(11): 147−156. DOI: 10.11686/cyxb2017043
[41] Wang S Y, Wang X Y, Liu Y, et al. Regulatory effect of graphene on growth and carbon/nitrogen metabolism of maize (Zea mays L.)[J]. Journal of the Science of Food and Agriculture, 2024, 104(3): 1572−1582. DOI: 10.1002/jsfa.13038
[42] de Avila S L, Condori-Apfata J A, Marcelino M M, et al. Nitrogen differentially modulates photosynthesis, carbon allocation and yield related traits in two contrasting Capsicum chinense cultivar[J]. Plant Science, 2019, 283: 224−237. DOI: 10.1016/j.plantsci.2019.02.014
[43] Cao L, Zou J N, Qin B, et al. Response of exogenous melatonin on transcription and metabolism of soybean under drought stress[J]. Physiologia Plantarum, 2023, 175(5): e14038. DOI: 10.1111/ppl.14038
[44] 徐冉, 陈松, 徐春梅, 等. 施氮量对籼粳杂交稻甬优1540产量和氮肥利用效率的影响及其机制[J]. 作物学报, 2023, 49(6): 1630−1642. Xu R, Chen S, Xu C M, et al. Effects of nitrogen fertilizer rates on grain yield and nitrogen use efficiency of japonica-indica hybrid rice cultivar Yongyou 1540 and its physiological bases[J]. Acta Agronomica Sinica, 2023, 49(6): 1630−1642.



 下载:
下载: