Research progress on the mechanism of rhizosphere microorganisms controlling soil-borne diseases under rational application of organic fertilizer
-
摘要:
土传病害因分布范围广、传播速度快且危害性严重,成为限制作物产质量提高的主要因素。近年来,多组学技术的发展揭示了合理配施有机肥下土壤微生物菌群结构和功能变化及其对土传病害的作用机理,对减少化肥施用和对农药的依赖,促进农业优质绿色发展具有重大意义。本文综述了合理配施有机肥对土传病害的防控效应,分析了合理配施有机肥下根际微生物调控土传病害的结构和功能响应,描述了调控根际微生物的土壤驱动因素,进一步从根际土壤微生物的直接作用、土壤微生物互作以及诱导植物免疫等方面,探究阐述了与根际微生物群落调控相关的抑病机理,探讨与展望了今后合理配施有机肥影响土传病害的重点研究方向,旨在通过施肥管理调控土壤微生物多样性和稳定性,进而促进土壤和植物健康。
Abstract:Soil-borne diseases have become a major factor limiting the improvement of crop yield and quality because of their wide distribution, rapid spread, and server harmfulness. In recent years, advances in multi-omics technologies have shed light on the changes of soil microbial community structure and function under rational application of organic fertilizers, as well as their mechanisms of inhibiting soil-borne diseases. These insights are of great significance to reduce chemical fertilizers and pesticides application and promote the agricultural green development. This paper reviewed the prevention and control effects of organic fertilizer application on soil borne diseases, analyzed the structural and functional responses of rhizosphere microorganisms in regulating soil borne diseases under such conditions, and described the soil driving factors of regulating rhizosphere microorganisms. Furthermore, the inhibition mechanisms related to rhizosphere microorganisms on soil-borne disease were summarized from the aspects such as the direct effects of rhizosphere soil microorganisms, soil microbial interactions, and induction of plant immunity. The article also discusses and outlines key future research directions on the impact of rational application of organic fertilizers on soil-borne diseases, aiming to regulate soil microbial diversity and stability through fertilization management, thereby promoting soil and plant health.
-
土传病害,顾名思义,是指由生活史中一部分或大部分存在于土壤中的病原物在适宜条件下侵染植物根部或茎部而引发的病害[1]。同时,栖息于土壤中的病原体生存能力和侵染能力均较强[2],并常年累积,引起作物根腐、黑胫、枯萎、猝倒等[3]。尤其伴随着高投入与高产出、单一化种植模式与复种指数增加,以及化学防治应用等现代集约化农业生产方式的快速发展,极易造成土壤理化性质恶化、微生物群落多样性破坏,以及病原体抗药性和药物残留等问题[4−5],进一步导致连作障碍现象,以及土传病害爆发日趋严重。土传病害成为危害全球作物生产的关键因子,严重制约着作物产量和质量的提高。有效防控土传病害,缓解连作障碍,是保障作物安全、培育健康土壤、提高农田生态系统多样性和稳定性,以及促进现代农业绿色可持续发展的重要需求[5−6]。
近年来,根际土壤微生物与土传病害发生相互关系成为研究热点之一。根际土壤微生物组成复杂与活性较高,在帮助作物抵御土传病原菌入侵方面具有重要作用,被称为植物的第二基因组[7−8]。尤其施肥措施影响着根际土壤微生物群落组成与结构差异[9]。研究发现,合理配施有机肥可显著提高根际微生物群落稳定性与减少病原菌丰度[10],并定向富集与调控土壤功能性微生物[11],达到控制土传病害与提高土壤健康的目的。同时,短期配施有机肥驱动的土壤微生物群落变化容易恢复[12],而长期配施有机肥4~5年后,土壤微生物群落会发生结构演替和功能演化,逐渐变为优势微生物发挥重要作用[13]。在化学肥料与化学药剂减施以及土传病害日益严重的背景下,合理配施有机肥成为防治作物土传病害、改良土壤与保护环境的重要措施,并具有广阔的应用前景。因此,有必要在厘清合理配施有机肥对土传病害防控影响的基础上,进一步探讨合理配施有机肥如何调控根际土壤微生物,实现降低土传病害发生的过程机制,对重构健康的农田生态系统以及提升农田生态系统服务功能等意义重大。
鉴于此,本文分析了合理配施有机肥对土传病害的防控效应,以及对根际土壤微生物群落组成和结构的影响,进一步剖析了合理配施有机肥如何驱动土壤微生物群落变化,并从根际土壤微生物直接作用、土壤–微生物互作以及诱导植物免疫等方面,探究合理配施有机肥防控土传病害的微生物功能作用机制,揭示合理配施有机肥通过调控根际微生物群落进而达到抑制土传病害发生的机理,最后指出今后研究的重点方向,以期为有机肥的合理施用以及土传病害的有效防控提供一定的理论参考和实践指导。
1. 合理配施有机肥对土传病害的防控效应
近年来,农作物土传病害成为限制作物产量和质量的重要因素之一。研究表明,不合理且长期单一的化肥投入会对土壤微生物群落造成不利影响,进而导致病原菌丰度增加和土传病害发生[14−16]。合理配施有机肥可改善土壤环境,对缓解土传病害与连作障碍以及维持土壤健康具有重要作用。合理配施有机肥显著降低了黄瓜连作障碍[17]及烟草[18]、番茄[19]青枯病与香蕉枯萎病[20−21]等病原菌数量与发病率。同时,长期合理配施有机肥[10, 22],以及短期的合理配施有机肥[23]、生物有机肥[24−26]、生物炭[27−28]、合成菌群[29]等均有利于诱导根际微生物群落变化,增加有益菌丰度,减少病原体丰度,降低土传病害发生。因此,合理配施有机肥有利于土壤微生物多样性维持,为调控根际土壤微生物群落与土壤微生态平衡提供基础,同时降低病原菌数量,保护植物免受病原菌侵害[30],为土传病害防控以及土壤、植物健康维护提供了巨大的潜力。
2. 合理配施有机肥下根际微生物群落结构和功能的响应
土壤微生物群落在调节植物健康方面具有重要影响与作用[31],是防止病原菌侵染与降低土传病害发生的生物屏障[32]。因此,维持土壤微生物多样性,提高有益菌比例,降低致病菌比例,对土壤健康具有重要作用。
2.1 根际土壤细菌和真菌群落组成和功能变化
土壤微生物群落高度复杂,其中细菌和真菌是土壤优势微生物,是维持微生物群落稳定和宿主健康的重要因素,尤其细菌和真菌间的相互作用塑造了作物相关微生物群落多样性和稳定性[33],保护作物免受病原菌侵害,提高作物健康程度。在微生物群落组成多样性方面,合理配施有机肥不仅有利于提高酸性土壤pH,而且导致腐生真菌被孢霉属(Mortierella)和Pseudaleuria的相对丰度显著增加,对提高植物抗逆性具有积极影响[34],同时显著增加富营养型细菌和植物有益真菌含量抑制病原微生物[15, 35]。合理配施生物有机肥不仅可凭借其本身的拮抗微生物来抵御病原菌,还可通过改变根际土壤微生物群落有益菌丰度,如激发土壤土著假单胞菌等有益微生物[21],增加细菌群落芽孢杆菌等丰度[24]以及鞘氨醇菌属、黄杆菌属等相对丰度[36],增加真菌群落腐质霉属等相对丰度[26],减轻土传病害发生。合理配施生物炭增加了芽孢杆菌属、土微菌属和一些真菌丰度,扰动根际有益微生物组变化[27−28, 37],对病原菌生长繁殖具有抑制作用。在微生物群落稳定性方面,配施有机肥量显著影响土壤细菌群落结构,适量有机肥能够促进形成较为复杂且稳定的细菌网络结构,从而有效抵御病原菌的侵染;而高量有机肥则降低土壤细菌多样性等[38]。同时,短时间配施有机肥主要是激活土著微生物群落,而外源微生物一段时间后会逐渐消失[39];相比之下,长达10年的有机替代才可显著提高土壤真菌网络的复杂度[40],抵御土传病害发生。总之,合理配施有机肥可增加农田根际微生物活性、数量等多样性与复杂性,减少有害生物数量,降低土传病害发生。值得注意的是,长期合理配施有机肥可激活更多有益和耐受的微生物[10],持续输入有机肥可优化土壤微生物群落结构,培养土著优势微生物,提高作物抵抗力,减轻土传病害发生。
合理配施有机肥提高了土壤的多功能性,而这种多功能性与土壤细菌和真菌的多样性呈正相关[41]。研究表明,长期配施有机肥或生物炭,能够调节根际土壤功能微生物组,增强与碳、氮、磷、硫循环相关的有机质降解、硝化及有机磷矿化等生态系统多功能性,从而提升农业生产效率[42]。不同施肥模式下,土壤有机碳、有效磷、真菌、粘粒含量与阳离子交换量是土壤健康评价的关键因子,且长期配施有机肥通过影响土壤碳循环、养分循环、生物多样性维持、缓冲过滤能力等功能提升土壤健康水平[43]。另一方面,配施生物有机肥可诱导鞘氨醇单胞菌科和黄单胞菌科细菌在根际富集,这些细菌能够产生与病害抑制相关的次生代谢物,显著增加非核糖体肽合成酶(NRPS)基因丰度[36],从而产生特定抑制。所以,合理配施有机肥可提高根际土壤微生物群落多样性和功能性,改善植物健康。
2.2 根际土壤原生生物群落组成和功能变化
原生生物是土壤微生物群落中最易被忽视的部分,作为“动态枢纽”在土壤管理和增强微生物群之间提供杠杆作用。研究表明,根际原生动物群落是决定作物健康的关键指标,在抵御土传病害方面发挥重要作用[44]。目前,关于施肥对土壤原生生物群落组成和功能影响报道较少。Xiong等[45]报道配施有机肥增加了食细菌型、杂食型、光能营养型等原生生物相对丰度,对植物健康具有显著影响。Guo等[46]跟踪了配施生物有机肥导致微食性的丝足虫类原生动物增加,进一步发现捕食型原生生物和芽孢杆菌相互作用,增加了次生代谢物Q基因如非核糖体肽合成酶基因丰度,及抗生素和脂肽抑制病原体,降低真菌性与细菌性病害[47−48]。长期定位试验研究表明,施肥对土壤原生生物群落组成无显著影响,但显著提高了原生生物功能群的相对丰度,如寄生生物顶复门和卵菌门的相对丰度以及消费者型的侧口纲、变形虫纲等[49]。因此,建议加强合理配施有机肥下土壤原生生物群落变化研究,为土壤和植物健康以及新的农业管理措施提供理论参考依据,服务土壤生态系统,降低土传病害发生。
2.3 根际土壤病毒组成和功能变化
病毒是地球上数量最多和种类最丰富的生命体。我国红壤地区施肥对根际土壤病毒群落组成的影响无显著差异,其中有尾噬菌体目是主要病毒类群[50]。一般侵染细菌和古菌原核微生物的病毒称为噬菌体,因此噬菌体对土传细菌病害的防控成为了目前的研究热点。噬菌体进行组合调控土壤菌群结构,增加根际有益细菌群落结构和多样性,实现靶向防控土传青枯菌[51],还可以通过靶向侵染有益土著细菌,间接促进土壤青枯菌降低[52]。将噬菌体与有机肥联合配施可显著提高拟杆菌门相对丰度,降低青枯病发生率[53]。目前,关于土壤病毒对施肥响应的研究较少,因此可从合理配施有机肥对土壤病毒群落组成、功能的影响,以及其在调控土传病害、促进植物生长和提升土壤健康方面的作用上,开展进一步的研究。
合理配施有机肥有助于改善根际土壤细菌、真菌、原生生物以及病毒等微生物群落的组成和促进其功能(表1),刺激有益菌富集,增加土壤微生物群落的稳定性等,提高土壤和植物健康程度,是农业废弃资源利用与促进农业可持续发展的关键举措。
表 1 根际有益和病原菌对有机肥的响应及功能Table 1. Response of beneficial rhizosphere microorganisms to organic fertilizers and the resulted functions有机肥
Organic
fertilizer试验年限
Experimental
years寄主植物
Host
plant根际微生物响应 Response of rhizosphere microorganisms 功能
Function参考文献
Reference病原菌
Pathogen有益菌
Beneficial microorganisms猪粪
Pig manure≥25 小麦
Wheat
玉米Maize真菌群落减少
Less fungal communities被孢霉Mortierella和Pseudaleuria富集
Saprophytic fungi of Mortierella and
Pseudaleuria were enriched提高抗性
Enhancing resistance[34] 有机肥
Organic fertilizer30 小麦
Wheat镰刀菌、青霉菌数量降低
Fusarium, and
Penicillium decreased富营养型细菌和有益真菌增加
Increased trophic bacteria and beneficial fungi导致真菌类病害
Cause fungus disease[15,
35]牛粪
Cow
manure35 小麦
Wheat病原菌丰度下降
Pathogens abundance
decreased细菌和真菌群落稳定性增加
Enhanced stability of bacterial and fungal communities抗真菌类病害
Anti-fungus disease[10] 有机肥
Organic fertilizer1 水稻
Paddy rice原生生物病原体
数量降低
Protozoan pathogens
numbers decreased食细菌型、杂食型、光能营养型等原生生物功能类群的丰度增加
Increased abundance of protist functional groups
such as bacterivorous, omnivorous and light nutrition and so on提高抗病害能力
Enhancing resistance
to disease[45] 有机肥
Organic fertilizer9 高粱
Sorghum
玉米
Maize改变原生生物消费者和寄生虫功能群
Functional communities of
protozoan consumer and
parasite changed显著改变特定功能群微生物群落
Significantly change the composition of
the specific functional microbial communities生态功能
Ecological function[49] 生物有机肥
Bio-organic fertilizer9 番茄
Tomato
香蕉
Banana病原菌数量减少
Pathogens numbers
decreased原生生物和芽孢杆菌属丰度, 尤其次级代谢物Q基因相对丰度增加
Increasing the relative bundances of protozoa and Bacillus, especially that of the secondary metabolite Q genes抗枯萎病、
青枯病
Anti-Fusarium, and bacterial wilt[47,
48]生物有机肥
Bio-organic fertilizer1 香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
numbers decreased激发土著芽孢杆菌等有益菌丰度,重塑土壤微生物群落组成与功能
Stimulating indigenous bacillus abundance and remodeling soil microbial community composition and function抗枯萎病
Anti-Fusarium wilt disease[24] 木霉有机肥
Trichoderma organic fertilizer3 季
3 seasons香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased有益木霉与土著腐质霉属抑制真菌,形成益生“菌团”协同作用
Trichoderma and indigenous Humicola inhibiting fungi and the probiotic ‘flora’ formation synergistic effect抗枯萎病
Anti-Fusarium wilt disease[26] 生物有机肥
Bio-organic fertilizer10 香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased重塑细菌群落并刺激特定益生假单胞菌增加,通过生物膜形成防控
Reshape bacterial community and stimulate specific probiotic Pseudomonas populations and biofilm formation to prevent抗枯萎病
Anti-Fusarium wilt disease[21] 生物有机肥
Bio-organic fertilizer长期
Long-
term番茄
Tomato青枯菌数量降低
Ralstonia solanacearum
decreased刺激鞘氨醇单孢菌科和黄单胞菌科富集,显著增加非核糖体肽合成酶基因丰度
Enriching Sphingomonosporaceae and Xanthomonadaceae enrichment, and significant increasing of non-ribosomal peptide synthase (NRPS) gene abundance抗青枯病
Anti-bacterial wilt[36] 噬菌体有机肥
Bacteriophage organic fertilizer1 番茄
Tomato青枯菌数量降低
Ralstonia solanacearum
decreased增加有益细菌群落数量和多样性
Increased number and diversity of the beneficial bacteria communities抗青枯病
Anti-bacterial wilt[51−53] 生物炭
Biochar1 人参
Ginseng镰刀菌属丰度降低19%~35%
Fusarium abundance decreased by 19%−35%Burholderia, Pesudomonas及丛枝菌根真菌(AMF)丰度增加,改善土壤团聚体功能,复杂性增强
The abundance of Burholderia, Pesudomonas and arbuscular mycorrhizal fungi (AMF) increased, the function of soil aggregates was improved, and the complexity of community was significantly enhanced抗真菌类病害
Anti-fungus disease[28] 生物炭
Biochar1 番茄
Tomato尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased诱导假单胞菌等丰度增加与生物膜形成,防控病原菌
Induce Pseudomonas abundance increasing and biofilm formation to prevent and control pathogenic bacteria抗枯萎病
Anti-Fusarium wilt disease[37] 生物炭
Biochar3 大豆
Soybean
玉米
Maize镰刀菌属和黑粉菌属丰度
降低
Fusarium and Ustilago
abundance decreased增加芽孢杆菌和土微菌属等丰度
Increasing the abundance of Bacillus and soil microbacteria抗腐病、黑霉病
Anti-rot and black mold disease[27] 3. 合理配施有机肥下根际微生物群落组成和变化的土壤驱动因素
根际土壤微生物群落组成和功能对土壤健康至关重要。然而土壤微生物群落对环境条件变化很敏感,尤其微生物与病原菌之间关系受各种环境因素波动影响,从而影响土传病害的发生。土壤理化特性、土壤微生物多样性和功能共同介导了植物防御和作物吸收能力对有机肥的响应[54]。全国20年的研究表明,土壤细菌多样性对施肥的响应存在区域差异[49],受生态环境影响[55]。
物理、化学、生化和生物因素均影响根际土壤生态环境,土壤类型是决定根际细菌群落组成的主要物理因素[56]。但长期配施有机肥仍可增强土壤团聚体的多功能性,驱动土壤微生物组成和功能的演变[57]。团聚体颗粒大小影响土壤中细菌和真菌群落结构[58],配施生物有机肥通过改变微团聚体数量和占比,增加土壤微生物Metallibacterium、Aggregicoccus、Steroidobacter、 Gemmatimonas与Salinithrix等相对丰度,进而抑制番茄青枯病发生[59]。
土壤化学性质是影响根际生态环境变化的重要因子。通过Meta方法全面分析我国长期定位试验中有关合理配施有机肥对土传病害的防控结果,发现土壤pH变化是降低土传病害发病率的主要原因[60−61]。配施有机肥可缓解土壤酸化,大大降低阻止病原体入侵的能力[62]。pH变化会导致土壤氮循环功能微生物群落的稳定性变低[63]。除了土壤pH外,前人发现高量配施有机肥还会引起土壤总碳、总氮、有效磷、碳磷比和氮磷比的变化,进而显著影响土壤细菌群落;而合理配施有机肥对土壤细菌多样性的影响较小[38]。但是Li等[43]研究表明,长期施用有机肥维持了相对较高水平的多功能性,如较高的土壤有机碳和磷的有效性,显著增加土壤微生物真菌、细菌、放线菌等群落多样性,改善土壤健康。另外,合理配施有机肥下,土壤pH及有机质、氮、磷、钾含量是原生生物功能组成的主要驱动因素,且与土壤pH值密切相关[45]。值得注意的是,针对不同作物,配施有机肥对土壤微生物群落影响的驱动因子不同,配施有机肥下小麦主要通过土壤pH影响细菌和真菌群落,而水稻主要通过土壤有机碳显著影响细菌组装[64]。
土壤酶活性可归类于根际生态变化的生化驱动因子。合理配施有机肥的土壤β-葡萄糖苷酶和蔗糖酶活性影响土壤微生物群落结构和功能[49],土壤pH变化通过影响土壤酸性磷酸单酯酶活性进而影响土壤细菌和真菌群落[64]。根际土壤微生物群落在生态系统功能中的作用毋庸置疑。其中,微生物,特别是关键物种和主导类群,是驱动土壤养分循环、土壤健康等微生物组结构和功能的关键因素[65]。但有研究发现,长期配施有机肥下,土壤中稀有微生物类群蓝藻Cyanobacteria和球囊菌门Glomeromycota是土壤微生物多功能的主要驱动因素[66]。因此,土壤理化性质以及稀有微生物类群变化是响应合理配施有机肥调控根际土壤微生物群落与功能的驱动因素,并且这一结果与根际微生物组中高于96.5%的绝大部分类群由土壤性质决定的研究报道相似[67],可通过合理的土壤管理措施精准调控根际微生物结构和功能,实现农业绿色发展。
4. 合理配施有机肥影响根际微生物防控土传病害作用机理
4.1 根际微生物对病原菌抑制机理
4.1.1 捕食、重寄生抑制病原菌
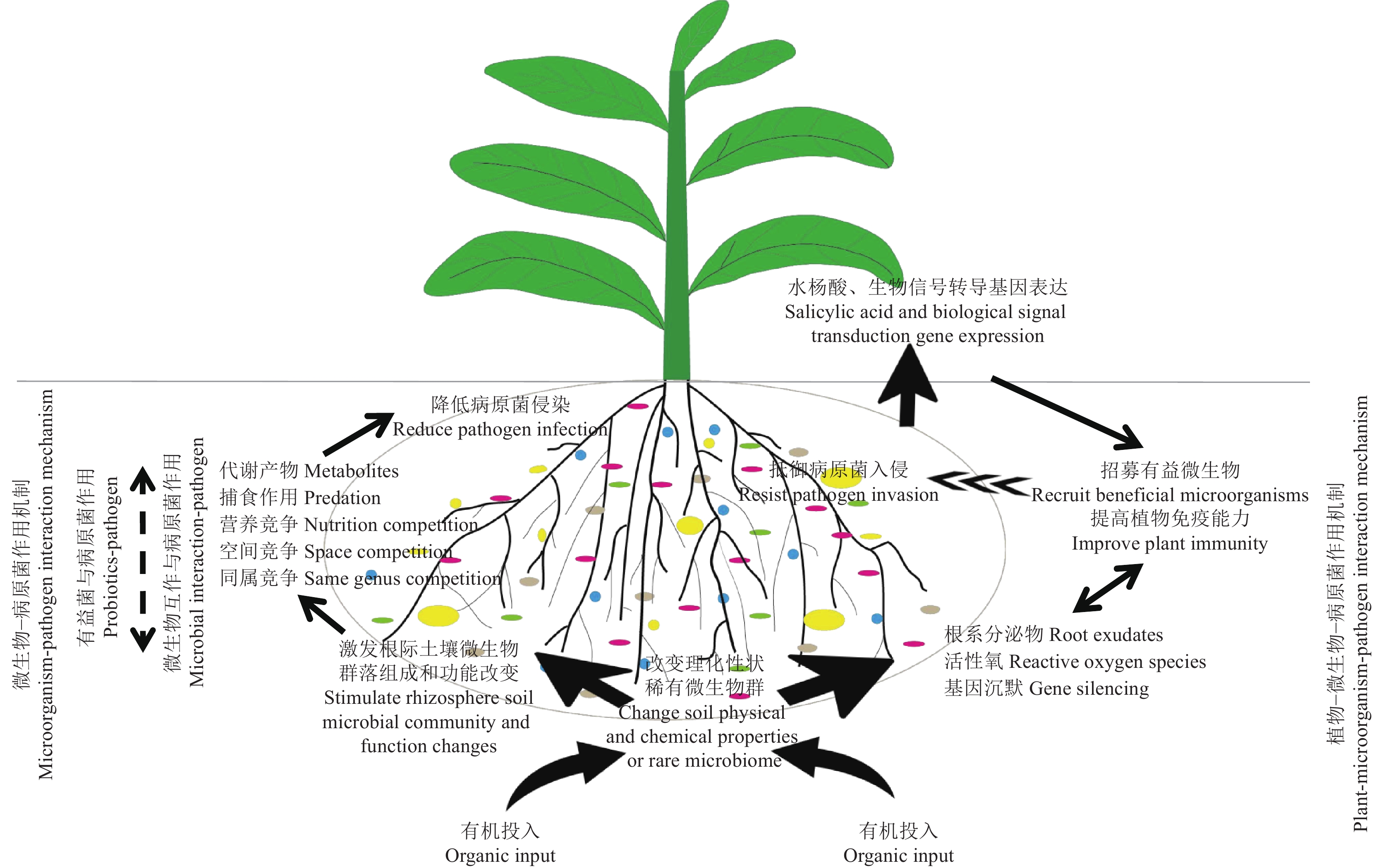
自然界中一般所有生物间都存在捕食与被捕食关系,微生物之间的捕食关系是指一种微生物以另一种微生物为猎物进行吞食和消化的现象,彼此消长,维持生态平衡。农田土壤生态系统中,粘细菌可通过直接捕食降低土壤中尖孢镰刀菌含量[68],吞噬型原生生物特别是微食性的丝足虫类也可通过直接捕食控制病原菌的生长。合理配施有机肥显著提高了土壤粘细菌属丰度[69],改变微食性的丝足虫类相对丰度[70],抑制土传病害爆发,改善土壤健康,提高农田作物产量。重寄生,顾名思义,就是病原菌被其他微生物寄生的现象,常常以吸附、缠绕、侵入、消解等形式寄生。哈茨木霉是著名的生防菌株,通过病原体识别信号转导、水解酶、转运蛋白、抗菌以及防御相关功能基因表达显著上调等调控真菌寄生过程,导致病原菌菌丝消解[71],为生物防治提供了思路。
4.1.2 空间、营养竞争抑制病原菌
微生物间存在资源争夺和空间争夺等相互抑制作用。连作土壤中添加有机肥增加了真菌烟曲霉和茄病镰刀菌丰度,通过营养竞争或茄病镰刀菌同属竞争等抑制了尖孢镰刀菌的生长[20]。长期配施有机肥可增加红壤中假单胞菌属和厌氧绳菌属丰度,促进红壤中Fe(II)氧化[72],而铁载体是根际微生物与病原菌争夺根际核心稀缺铁素资源的秘密武器[73]。配施噬菌体组合可重新调整根际土壤菌群结构,恢复群落多样性,增加群落有益菌丰度与病原菌生态竞争能力,防控土传病害发生[51]。
4.1.3 形成生物膜、抗生物质抑制病原菌
研究发现,配施的生物有机肥中功能微生物芽孢杆菌,可与土著有益假单胞菌相互作用,增强生物被膜的形成,潜在触发微生物根系定殖和抗病性[21]。另外,许多功能性微生物假单胞菌属、芽孢杆菌属、木霉属等都可产生抗生素包括脂肽[74]、吩嗪类[75]等,对病原体具有直接抑制作用。近几年,合理配施有机肥的抑病作用研究主要集中在生防菌的拮抗作用,除此之外,根际细菌群落组成和功能的响应也倍受关注。合理配施生物有机肥增加了常驻携带抗菌生物合成基因簇(BGCs)[31, 76]以及富含次级代谢产物合成基因(Q基因)的微生物显著富集[36, 47],抑制病原菌。此外,根际微生物能够产生内切葡聚糖酶、几丁质酶和蛋白酶等,这些酶在病原体的细胞壁降解中起着重要作用[77]。
4.1.4 根际微生物交流协同增强抑制病原菌
自然微生物群落中,微生物的性状需要通过微生物之间的交流与合作才能表现出来,促进作物健康。假单胞菌抗真菌抗生素的产生需要土壤中溶杆菌触发[78]。目前合成菌群的研究,也证实了群落物种间互作增强预防土传病害发生。一方面,群落之间相互作用,更善于与病原菌进行有限土壤资源竞争,抑制疾病;另一方面,单个微生物种无法合成某些抗生素对病原菌产生拮抗作用,需微生物间相互作用激发而发挥特定功能,研究发现赤霉菌只有与枯草芽孢杆菌或放线菌相互作用才会促进合成原苷酸,抑制大丽轮枝霉[79]。另外,有机物料输入对土著菌群造成影响,导致土著微生物间或接种功能微生物与土著微生物间积极相互作用与影响,抑制病原体,提高植物健康程度。已有研究表明,生物有机肥通过刺激土著有益微生物富集以及代谢产物产生,共同防御病原菌入侵[21];并且有些微生物的代谢产物被抑制,如土著贝莱斯芽孢杆菌感知假单胞菌的铁载体提高代谢产物的产生,增强抗菌活性[80]。因此,微生物之间交流作用有助于加强土壤病原体抑制。然而,关于微生物间相互作用如何促进对病原菌抑制的具体机制,仍需进一步广泛深入的研究。比如,微生物之间可能通过相互刺激产生新型化合物等方式来抑制病原菌[79],这为生物防治更有效地抑制病原菌提供了理论依据。
4.2 植物与根际土壤微生物相互作用对病原菌的抑制机理
4.2.1 植物生理变化提高根际土壤微生物群落活性,从而抑制病原菌
研究发现,在生物胁迫或非生物胁迫条件下,植物进行抗逆代谢和免疫反应,调控根系微生物组,促进植物健康。但是,植物不同发育阶段调控表达功能基因不同,生长前期促进微生物生防相关基因表达,生长后期促进促生相关基因表达[81]。生长前期通过植物生理变化,根系分泌物有利于募集有益微生物抵御病害[82],例如蔗糖的渗出增强了枯草芽孢杆菌和假单胞菌定殖,保护植物免受镰刀菌Fusarium和葡萄孢属Botrytis危害[83]。合理配施生物炭改变了番茄根系分泌物,促进招募特殊的PGPR假单胞菌株保护番茄[37],同时激发土壤微生物的抗生素合成等途径降低土传病害[84] 。黄瓜根系分泌苏糖酸和赖氨酸招募芽孢杆菌和鞘氨醇单胞菌,诱导根部维持高ROS水平,保护其免受病原体感染[85]。除了根系分泌物外,合理配施有机肥还促进植物水杨酸(SA)的积累[86−87],增强植物体内氧化和生物胁迫信号转导通路相关基因的表达[9],驱动根际微生物群落组装,响应并抵御病原真菌的入侵。
4.2.2 根际土壤微生物群落改变诱导植物免疫抑制病原菌
除宿主生理诱导微生物群落变化抵御病原菌外,微生物通过活性氧和氮氧化物爆发、激发植物防御激素信号启动、抗菌代谢产物释放等导致植物防御反应[88]。生物有机肥的有益微生物也能够激发植物活性氧爆发,且有益微生物比病原菌耐受更高浓度的活性氧[89],降低病害发生。此外,有益微生物还可通过影响植物水杨酸信号[90]、基因沉默机制[91]等调控植物免疫系统,抵御病原菌入侵。
合理配施有机肥影响根际微生物防控土传病害的多种作用机理见图1。根际微生物对病原菌的直接和间接抑制机理之间的相互作用也不容忽视,例如根际细菌对青枯菌帮手的抑制作用是抑制土传青枯病的决定因素[92]。因此,今后应拓展根际微生物对病原菌抑制作用研究,为施用有机肥抵御土传病害提供理论依据。
5. 结论与展望
合理配施有机肥可改变土壤性质与关键微生物类群等,驱动土壤微生物群落组装或高效定殖或激活植物抗性免疫系统,保护植物免受病原菌侵害。这些研究有助于调控和管理土壤微生物组,激发和增强土壤抑病能力,提高植物健康程度,为实现土壤健康的可持续农业管理提供实践依据。结合目前研究进展,如何进一步通过有效的管理措施,提高土壤免疫和植物抗病性仍是未来主要研究方向。
5.1 拓展微生物的互作研究,开发高效的微生物产品,抵御病原菌的侵染
协调的微生物群落可有效维护植物健康[93]。在根际土壤中,除了真菌和细菌外,还存在原生生物、古菌、藻类等微生物,因此需要深入探讨在合理配施有机肥的条件下,不同种类微生物间的相互作用对植物健康的影响;此外,植物根系内生菌构成了抵抗土传病害的第二道防线[94],有必要探索合理配施有机肥后根内微生物群落的组成和功能差异,及其抵抗病害的作用机制。研究发现,大丽轮枝菌会分泌一种“效应蛋白”操纵与逃避根际微生物组,促进病原菌入侵,间接影响植物健康[95]。因此,需要探索在合理配施有机肥的条件下,病原菌的生存策略以及诱导的抗病微生物对病害的抑制作用,为开发高效微生物产品与建立有效的促生防病农业管理措施提供理论支撑。
5.2 深入植物与微生物协同研究,筛选有益的调控化合物,降低病害发生
Valle等[96]研究表明,土壤有机物切断了黄酮类植物与微生物的联系,进一步增强了合理配施有机肥对植物根系分泌物产生的诱导作用,同时建立抗病土传微生物组,共同降低病害发生。此外,研究发现,外源添加谷氨酸重塑了微生物群落组成,增加了有益菌种群规模,从而降低病害发生[97]。因此,需进一步验证外源添加调控化合物对植物代谢组与微生物组的影响,为开发有益调控化合物抵御病害侵染的应用研究提供理论与实践基础。
5.3 优化配施有机肥措施与联用技术研究,防控土传病害
微生物对有机肥组成具有偏好性[98],因此,探索并选择适宜的有机肥种类,对于提升土壤微生物多样性和植物健康水平具有重要意义。同时,作物轮作与土著微生物接种更有利于抵抗病原菌侵染[99]。因此,应将这些策略分别与土壤熏蒸、土壤物理措施、栽培措施等联用,减轻土传病害,改善植物健康,从而为我国土传病害的有效防控以及土壤保育提供切实可行的生产改良方案。
-
表 1 根际有益和病原菌对有机肥的响应及功能
Table 1 Response of beneficial rhizosphere microorganisms to organic fertilizers and the resulted functions
有机肥
Organic
fertilizer试验年限
Experimental
years寄主植物
Host
plant根际微生物响应 Response of rhizosphere microorganisms 功能
Function参考文献
Reference病原菌
Pathogen有益菌
Beneficial microorganisms猪粪
Pig manure≥25 小麦
Wheat
玉米Maize真菌群落减少
Less fungal communities被孢霉Mortierella和Pseudaleuria富集
Saprophytic fungi of Mortierella and
Pseudaleuria were enriched提高抗性
Enhancing resistance[34] 有机肥
Organic fertilizer30 小麦
Wheat镰刀菌、青霉菌数量降低
Fusarium, and
Penicillium decreased富营养型细菌和有益真菌增加
Increased trophic bacteria and beneficial fungi导致真菌类病害
Cause fungus disease[15,
35]牛粪
Cow
manure35 小麦
Wheat病原菌丰度下降
Pathogens abundance
decreased细菌和真菌群落稳定性增加
Enhanced stability of bacterial and fungal communities抗真菌类病害
Anti-fungus disease[10] 有机肥
Organic fertilizer1 水稻
Paddy rice原生生物病原体
数量降低
Protozoan pathogens
numbers decreased食细菌型、杂食型、光能营养型等原生生物功能类群的丰度增加
Increased abundance of protist functional groups
such as bacterivorous, omnivorous and light nutrition and so on提高抗病害能力
Enhancing resistance
to disease[45] 有机肥
Organic fertilizer9 高粱
Sorghum
玉米
Maize改变原生生物消费者和寄生虫功能群
Functional communities of
protozoan consumer and
parasite changed显著改变特定功能群微生物群落
Significantly change the composition of
the specific functional microbial communities生态功能
Ecological function[49] 生物有机肥
Bio-organic fertilizer9 番茄
Tomato
香蕉
Banana病原菌数量减少
Pathogens numbers
decreased原生生物和芽孢杆菌属丰度, 尤其次级代谢物Q基因相对丰度增加
Increasing the relative bundances of protozoa and Bacillus, especially that of the secondary metabolite Q genes抗枯萎病、
青枯病
Anti-Fusarium, and bacterial wilt[47,
48]生物有机肥
Bio-organic fertilizer1 香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
numbers decreased激发土著芽孢杆菌等有益菌丰度,重塑土壤微生物群落组成与功能
Stimulating indigenous bacillus abundance and remodeling soil microbial community composition and function抗枯萎病
Anti-Fusarium wilt disease[24] 木霉有机肥
Trichoderma organic fertilizer3 季
3 seasons香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased有益木霉与土著腐质霉属抑制真菌,形成益生“菌团”协同作用
Trichoderma and indigenous Humicola inhibiting fungi and the probiotic ‘flora’ formation synergistic effect抗枯萎病
Anti-Fusarium wilt disease[26] 生物有机肥
Bio-organic fertilizer10 香蕉
Banana尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased重塑细菌群落并刺激特定益生假单胞菌增加,通过生物膜形成防控
Reshape bacterial community and stimulate specific probiotic Pseudomonas populations and biofilm formation to prevent抗枯萎病
Anti-Fusarium wilt disease[21] 生物有机肥
Bio-organic fertilizer长期
Long-
term番茄
Tomato青枯菌数量降低
Ralstonia solanacearum
decreased刺激鞘氨醇单孢菌科和黄单胞菌科富集,显著增加非核糖体肽合成酶基因丰度
Enriching Sphingomonosporaceae and Xanthomonadaceae enrichment, and significant increasing of non-ribosomal peptide synthase (NRPS) gene abundance抗青枯病
Anti-bacterial wilt[36] 噬菌体有机肥
Bacteriophage organic fertilizer1 番茄
Tomato青枯菌数量降低
Ralstonia solanacearum
decreased增加有益细菌群落数量和多样性
Increased number and diversity of the beneficial bacteria communities抗青枯病
Anti-bacterial wilt[51−53] 生物炭
Biochar1 人参
Ginseng镰刀菌属丰度降低19%~35%
Fusarium abundance decreased by 19%−35%Burholderia, Pesudomonas及丛枝菌根真菌(AMF)丰度增加,改善土壤团聚体功能,复杂性增强
The abundance of Burholderia, Pesudomonas and arbuscular mycorrhizal fungi (AMF) increased, the function of soil aggregates was improved, and the complexity of community was significantly enhanced抗真菌类病害
Anti-fungus disease[28] 生物炭
Biochar1 番茄
Tomato尖孢镰刀菌数量降低
Fusarium oxysporum
number decreased诱导假单胞菌等丰度增加与生物膜形成,防控病原菌
Induce Pseudomonas abundance increasing and biofilm formation to prevent and control pathogenic bacteria抗枯萎病
Anti-Fusarium wilt disease[37] 生物炭
Biochar3 大豆
Soybean
玉米
Maize镰刀菌属和黑粉菌属丰度
降低
Fusarium and Ustilago
abundance decreased增加芽孢杆菌和土微菌属等丰度
Increasing the abundance of Bacillus and soil microbacteria抗腐病、黑霉病
Anti-rot and black mold disease[27] -
[1] 杨珍, 戴传超, 王兴祥, 李孝刚. 作物土传真菌病害发生的根际微生物机制研究进展[J]. 土壤学报, 2019, 56(1): 12−22. Yang Z, Dai C C, Wang X X, Li X G. Advance of research on rhizosphere microbial mechanisms of crop soil-borne fungal diseases[J]. Acta Pedologica Sinica, 2019, 56(1): 12−22.
[2] 魏勇, 高嵩涓, 曹卫东, 段廷玉. 绿肥影响农田土传病害的研究进展[J]. 草地学报, 2021, 29(8): 1605−1614. Wei Y, Gao S J, Cao W D, Duan T Y. Research progress on the influence of green manures on soil-borne diseases in farmlands[J]. Acta Agrestia Sinica, 2021, 29(8): 1605−1614.
[3] 李世东, 缪作清, 高卫东. 我国农林园艺作物土传病害发生和防治现状及对策分析[J]. 中国生物防治学报, 2011, 27(4): 433−440. Li S D, Miao Z Q, Gao W D. Challenges, opportunities and obligations in management of soil-borne plant diseases in China[J]. Chinese Journal of Biological Control, 2011, 27(4): 433−440.
[4] Saleem M, Hu J, Jousset A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health[J]. Annual Review of Ecology, Evolution and Systematics, 2019, 50(1): 145−168. DOI: 10.1146/annurev-ecolsys-110617-062605
[5] 朱永官, 彭静静, 韦中, 等. 土壤微生物组与土壤健康[J]. 中国科学: 生命科学, 2021, 51(1): 1−11. Zhu Y G, Peng J J, Wei Z, et al. Linking the soil microbiome to soil health[J]. Scientia Sinica (Vitae), 2021, 51(1): 1−11.
[6] 张俊伶, 张江周, 申建波, 等. 土壤健康与农业绿色发展: 机遇与对策[J]. 土壤学报, 2020, 57(4): 783−796. Zhang J L, Zhang J Z, Shen J B, et al. Soil health and agriculture green development: Opportunities and challenges[J]. Acta Pedologica Sinica, 2020, 57(4): 783−796.
[7] Berendsen R L, Pieterse C M J, Bakker P A H M. The rhizosphere microbiome and plant health[J]. Trends in Plant Science, 2012, 17(8): 478−486. DOI: 10.1016/j.tplants.2012.04.001
[8] Sanguin H, Sarniguet A, Gazengel K, et al. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture[J]. New Phytologist, 2009, 184(3): 694−707. DOI: 10.1111/j.1469-8137.2009.03010.x
[9] Chowdhury S P, Babin D, Sandmann M, et al. Effect of long-term organic and mineral fertilization strategies on rhizosphere microbiota assemblage and performance of lettuce[J]. Environmental Microbiology, 2019, 21(7): 2426−2439. DOI: 10.1111/1462-2920.14631
[10] Fan K K, Delgado-Baquerizo M, Guo X S, et al. Microbial resistance promotes plant production in a four-decade nutrient fertilization experiment[J]. Soil Biology and Biochemistry, 2020, 141: 107679. DOI: 10.1016/j.soilbio.2019.107679
[11] Wang L K, Li X F. Steering soil microbiome to enhance soil system resilience[J]. Critical Reviews in Microbiology, 2019, 45(6): 743−753.
[12] Lourenço K S, Suleiman A K A, Pijl A, et al. Resilience of the resident soil microbiome to organic and inorganic amendment disturbances and to temporary bacterial invasion[J]. Microbiome, 2018, 6(1): 142. DOI: 10.1186/s40168-018-0525-1
[13] Feng Y Z, Chen R R, Hu J L, et al. Bacillus asahii comes to the fore in organic manure fertilized alkaline soils[J]. Soil Biology and Biochemistry, 2015, 81: 186−194.
[14] Li P F, Liu M, Li G L, et al. Phosphorus availability increases pathobiome abundance and invasion of rhizosphere microbial networks by Ralstonia[J]. Environmental Microbiology, 2021, 23(10): 5992−6003.
[15] Hu X J, Liu J J, Wei D, et al. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of Northeast China[J]. Agriculture, Ecosystems and Environment, 2017, 248: 113−122. DOI: 10.1016/j.agee.2017.07.031
[16] Wu Y T, Kwak J H, Karst J, et al. Long-term nitrogen and sulfur deposition increased root-associated pathogen diversity and changed mutualistic fungal diversity in a boreal forest[J]. Soil Biology and Biochemistry, 2021, 155: 108163. DOI: 10.1016/j.soilbio.2021.108163
[17] 曲成闯, 陈效民, 张志龙, 等. 生物有机肥提高设施土壤生产力减缓黄瓜连作障碍的机制[J]. 植物营养与肥料学报, 2019, 25(5): 814−823. DOI: 10.11674/zwyf.18311 Qu C C, Chen X M, Zhang Z L, et al. Mechanism of bio-organic fertilizer on improving soil productivity for continuous cucumber in greenhouse[J]. Journal of Plant Nutrition and Fertilizers, 2019, 25(5): 814−823. DOI: 10.11674/zwyf.18311
[18] 尹兴盛, 包玲凤, 濮永瑜, 等. 减氮配施生物有机肥对植烟土壤特性及烟草青枯病的防效研究[J]. 中国农业科技导报, 2023, 25(7): 122−131. Yin X S, Bao L F, Pu Y Y, et al. Effects of chemical fertilizer reduction combined with bio-organic fertilization on tobacco soil characteristics and tobacco bacterial wilt control[J]. Journal of Agricultural Science and Technology, 2023, 25(7): 122−131.
[19] 杨天杰, 王玉鑫, 王佳宁, 等. 不同基质生物有机肥防控番茄土传青枯病及促生效果研究[J]. 土壤, 2021, 53(5): 961−968. Yang T J, Wang Y X, Wang J N, et al. Effects of different bioorganic fertilizers on tomato bacterial wilt and plant growth promotion[J]. Soils, 2021, 53(5): 961−968.
[20] Yuan X F, Hong S, Xiong W, et al. Development of fungal-mediated soil suppressiveness against Fusarium wilt disease via plant residue manipulation[J]. Microbiome, 2021, 9: 200. DOI: 10.1186/s40168-021-01133-7
[21] Tao C Y, Li R, Xiong W, et al. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression[J]. Microbiome, 2020, 8(1): 137−150. DOI: 10.1186/s40168-020-00892-z
[22] 王小兵, 骆永明, 李振高, 等. 长期定位施肥对红壤地区连作花生生物学性状和土传病害发生率的影响[J]. 土壤学报, 2011, 48(4): 725−730. DOI: 10.11766/trxb200910100444 Wang X B, Luo Y M, Li Z G, et al. Effects of long-term stationary fertilization experiment on incidence of soil-borne diseases and biological characteristics of peanut in continuous monocropping system in red soil area[J]. Acta Pedologica Sinica, 2011, 48(4): 725−730. DOI: 10.11766/trxb200910100444
[23] 朱志炎, 梁雪雁, 林凤玲, 等. 有机肥对香蕉枯萎病及土壤主要理化性质和微生物群落的影响[J]. 福建农业学报, 2021, 36(7): 806−816. Zhu Z Y, Liang X Y, Lin F L, et al. Effects of organic fertilizer on physicochemical properties and microflora of banana field infected by Fusarium wilt disease[J]. Fujian Journal of Agricultural Sciences, 2021, 36(7): 806−816.
[24] Deng X H, Zhang N, Shen Z Z, et al. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum[J]. npj Biofilms and Microbiomes, 2021, 7(1): 33. DOI: 10.1038/s41522-021-00204-9
[25] 胡英宏, 任泽广, 杨姝钰, 等. 生物有机肥对菠萝心腐病发生和土壤细菌群落结构的影响[J]. 应用与环境生物学报, 2022, 28(6): 1444−1451. Hu Y H, Ren Z G, Yang S Y, et al. Effects of bio-organic fertilizers on pineapple heart rot and bacterial community structure[J]. Chinese Journal of Applied and Environmental Biology, 2022, 28(6): 1444−1451.
[26] Tao C Y, Wang Z, Liu S S, et al. Additive fungal interactions drive biocontrol of Fusarium wilt disease[J]. New Phytologist, 2023, 238(3): 1198−1214. DOI: 10.1111/nph.18793
[27] Yao Q, Liu J, Yu, Z, et al. Changes of bacterial community compositions after three years of biochar application in a black soil of Northeast China[J]. Applied Soil Ecology, 2017, 113: 11−21. DOI: 10.1016/j.apsoil.2017.01.007
[28] Liu C, Xia R, Tang M, et al. Improved ginseng production under continuous cropping through soil health reinforcement and rhizosphere microbial manipulation with biochar: A field study of Panax ginseng from Northeast China[J]. Horticulture Research, 2022, 9: uhac108. DOI: 10.1093/hr/uhac108
[29] Zhou X, Liu L L, Zhao J, et al. High carbon resource diversity enhances the certainty of successful plant pathogen and disease control[J]. New Phytologist, 2023, 237(4): 1333−1346. DOI: 10.1111/nph.18582
[30] Sun R B, Dsouza M, Gilbert J A, et al. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter[J]. Environmental Microbiology, 2016, 18(12): 5137−5150. DOI: 10.1111/1462-2920.13512
[31] Poppeliers S W M, Sánchez -Gil J J, de Jonge R. Microbes to support plant health: Understanding bioinoculant success in complex conditions[J]. Current Opinion in Microbiology, 2023, 73: 102286. DOI: 10.1016/j.mib.2023.102286
[32] Qiao Y, Wang T, Huang Q, et al. Core species impact plant health by enhancing soil microbial cooperation and network complexity during community coalescence[J]. Soil Biology and Biochemistry, 2024, 188: 109231. DOI: 10.1016/j.soilbio.2023.109231
[33] Getzke F, Thiergart T, Hacquard S. Contribution of bacterial-fungal balance to plant and animal health[J]. Current Opinion in Microbiology, 2019, 49: 66−72. DOI: 10.1016/j.mib.2019.10.009
[34] Duan Y, Chen L, Zhang J B, et al. Long-term fertilization reveals close associations between soil organic carbon composition and microbial traits at aggregate scales[J]. Agriculture, Ecosystems and Environment, 2021, 306: 107169. DOI: 10.1016/j.agee.2020.107169
[35] Hu X J, Liu J J, Dan W, et al. Soil bacterial communities under different long-term fertilization regimes in three locations across the black soil region of Northeast China[J]. Pedosphere, 2018, 28(5): 751−763. DOI: 10.1016/S1002-0160(18)60040-2
[36] Deng X H, Zhang N, Li Y C, et al. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities[J]. New Phytologist, 2022, 235(4): 1558−1574. DOI: 10.1111/nph.18221
[37] Jin X, Bai Y, Khashi U Rahman M, et al. Biochar stimulates tomato roots to recruit a bacterial assemblage contributing to disease resistance against Fusarium wilt[J]. iMeta, 2022, 1(3): e37. DOI: 10.1002/imt2.37
[38] Liu H Y, Huang X, Tan W F, et al. High manure load reduces bacterial diversity and network complexity in a paddy soil under crop rotations[J]. Soil Ecology Letters, 2020, 2: 104−119. DOI: 10.1007/s42832-020-0032-8
[39] Semenov M V, Krasnov G S, Semenov V M, et al. Does fresh farmyard manure introduce surviving microbes into soil or activate soil-borne microbiota[J]. Journal of Environmental Management, 2021, 294: 113018. DOI: 10.1016/j.jenvman.2021.113018
[40] Ji L F, Ni K, Wu Z D, et al. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization[J]. Biology and Fertility of Soils, 2020, 56: 633−646. DOI: 10.1007/s00374-020-01439-y
[41] Chen Q L, Ding J, Zhu D, et al. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils[J]. Soil Biology and Biochemistry, 2019, 141: 107686.
[42] Hu W, Zhang Y P, Rong M, et al. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality[J]. Biochar, 2024, 6(1): 3. DOI: 10.1007/s42773-023-00296-w
[43] Li X, Lei Q, Huang Y P, et al. Manuring improves soil health by sustaining multifunction at relatively high levels in subtropical area[J]. Agriculture, Ecosystems & Environment, 2023, 353: 108539.
[44] 韦中, 宋宇琦, 熊武, 等. 土壤原生动物: 研究方法及其在土传病害防控中的作用[J]. 土壤学报, 2021, 58(1): 14−22. Wei Z, Song Y Q, Xiong W, et al. Soil protozoa: Research methods and roles in the biocontrol of soil-borne diseases[J]. Acta Pedologica Sinica, 2021, 58(1): 14−22.
[45] Xiong W, Jousset A, Guo S, et al. Soil protist communities form a dynamic hub in the soil microbiome[J]. The ISME Journal, 2018, 12(2): 634−638. DOI: 10.1038/ismej.2017.171
[46] Guo S, Xiong W, Hang X N, et al. Protists as main indicators and determinants of plant performance[J]. Microbiome, 2021, 9: 64. DOI: 10.1186/s40168-021-01025-w
[47] Guo S, Tao C Y, Jousset A, et al. Trophic interactions between predatory protists and pathogen-suppressive bacteria impact plant health[J]. The ISME Journal, 2022, 16(8): 1932−1943. DOI: 10.1038/s41396-022-01244-5
[48] Guo S, Jiao Z X, Yan Z G, et al. Predatory protists reduce bacteria wilt disease incidence in tomato plants[J]. Nature Communications, 2024, 15(1): 829. DOI: 10.1038/s41467-024-45150-0
[49] Sun A Q, Jiao X Y, Chen Q L, et al. Fertilization alters protistan consumers and parasites in crop-associated microbiomes[J]. Environmental Microbiology, 2021, 23(4): 2169−2183. DOI: 10.1111/1462-2920.15385
[50] Bi L, Yu D T, Du S, et al. Diversity and potential biogeochemical impacts of viruses in bulk and rhizosphere soils[J]. Environmental Microbiology, 2021, 23(2): 588−599. DOI: 10.1111/1462-2920.15010
[51] Wang X F, Wei Z, Yang K M, et al. Phage combination therapies for bacterial wilt disease in tomato[J]. Nature Biotechnology, 2019, 37(12): 1513−1520. DOI: 10.1038/s41587-019-0328-3
[52] Yang K M, Wang X F, Hou R, et al. Rhizosphere phage communities drive soil suppressiveness to bacterial wilt disease[J]. Microbiome, 2023, 11(1): 16. DOI: 10.1186/s40168-023-01463-8
[53] 王佳宁, 王玉鑫, 侯如娇, 等. 噬菌体鸡尾酒联合生物有机肥防控番茄青枯病的效果研究[J]. 微生物学通报, 2021, 48(9): 3194−3204. Wang J N, Wang Y X, Hou R J, et al. Biocontrol of phage cocktail combined with bio-organic fertilizer on tomato bacterial wilt[J]. Microbiology China, 2021, 48(9): 3194−3204.
[54] Shu X Y, Jia H, Zhou Z H, et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis[J]. Science of the Total Environment, 2022, 829: 154627. DOI: 10.1016/j.scitotenv.2022.154627
[55] Feng Y Z, Manuel D B, Zhu Y G, et al. Responses of soil bacterial diversity to fertilization are driven by local environmental context across China[J]. Engineering, 2022, 12(5): 164−170.
[56] Yeoh Y K, Dennis P G, Paungfoo-Lonhienne C, et al. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence[J]. Nature Communications, 2017, 8(1): 215. DOI: 10.1038/s41467-017-00262-8
[57] Han S, Manuel D B , Luo X S, et al. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality[J]. Soil Biology and Biochemistry, 2021, 154: 108143.
[58] Liao H, Zhang Y C, Zuo Q Y, et al. Contrasting responses of bacterial and fungal communities to aggregate-size fractions and long-term fertilizations in soils of northeastern China[J]. Science of the Total Environment, 2018, 635: 784−792. DOI: 10.1016/j.scitotenv.2018.04.168
[59] Dong M H, Zhao M L, Shen Z Z, et al. Biofertilizer application triggered microbial assembly in microaggregates associated with tomato bacterial wilt suppression[J]. Biology and Fertility of Soils, 2020, 56: 551−563. DOI: 10.1007/s00374-020-01459-8
[60] 申云鑫, 施竹凤, 唐加菜, 等. 有机物料输入对作物土传病害防控作用的Meta分析[J]. 植物营养与肥料学报, 2023, 29(8): 1495−1506. DOI: 10.11674/zwyf.2023016 Shen Y X, Shi Z F, Tang J C, et al. Meta-analysis on soil-borne crop diseases suppression by organic material input[J]. Journal of Plant Nutrition and Fertilizers, 2023, 29(8): 1495−1506. DOI: 10.11674/zwyf.2023016
[61] Feng M M, Adams J M, Fan K K, et al. Long-term fertilization influences community assembly processes of soil diazotrophs[J]. Soil Biology and Biochemistry, 2018, 126: 151−158. DOI: 10.1016/j.soilbio.2018.08.021
[62] Li X G, Chen D L, Carrion V J, et al. Acidification suppress the natural capacity of soil microbiome to fight pathogenic Fusarium infections[J]. Nature Communications, 2023, 14(1): 5090. DOI: 10.1038/s41467-023-40810-z
[63] Sun R B, Wang D Z, Guo Z B, et al. Combined application of organic manure and chemical fertilizers stabilizes soil N-cycling microflora[J]. Soil Ecology Letters, 2023, 5(3): 220165. DOI: 10.1007/s42832-022-0165-z
[64] Xie Y N , Ouyang Y, Han S, et al. Crop rotation stage has a greater effect than fertilisation on soil microbiome assembly and enzymatic stoichiometry[J]. Science of the Total Environment, 2022, 815: 152956.
[65] Banerjee S, Schlaeppi K, van der Heijden M G A. Keystone taxa as drivers of microbiome structure and functioning[J]. Nature Reviews Microbiology[J]., 2018, 16(9): 567−576. DOI: 10.1038/s41579-018-0024-1
[66] Chen Q L, Ding J, Zhu D, et al. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils[J]. Soil Biology and Biochemistry, 2020, 141: 107686. DOI: 10.1016/j.soilbio.2019.107686
[67] Xun W B, Liu Y P, Ma A Y, et al. Dissection of rhizosphere microbiome and exploiting strategies for sustainable agriculture[J]. New Phytologist, 2024, 242(6): 2401−2410. DOI: 10.1111/nph.19697
[68] Ye X F, Li Z K, Luo X, et al. A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community[J]. Microbiome, 2020, 8: 49. DOI: 10.1186/s40168-020-00824-x
[69] Wang W H, Wang N, Dang K K, et al. Long-term nitrogen application decreases the abundance and copy number of predatory myxobacteria and alters the myxobacteria community structure in the soil[J]. Science of the Total Environment, 2020, 708: 135114. DOI: 10.1016/j.scitotenv.2019.135114
[70] Xiong W, Song Y Q, Yang K M, et al. Rhizosphere protists are key determinants of plant health[J]. Microbiome, 2020, 8: 27. DOI: 10.1186/s40168-020-00799-9
[71] Wang Y P, Zhu X C, Wang J, et al. Identification of mycoparasitism-related genes against the phytopathogen Botrytis cinerea via transcriptome analysis of Trichoderma harzianum T4[J]. Journal of Fungi, 2023, 9(3): 324. DOI: 10.3390/jof9030324
[72] Wen Y L, Xiao J, Liu F F, et al. Contrasting effects of inorganic and organic fertilisation regimes on shifts in Fe redox bacterial communities in red soils[J]. Soil Biology and Biochemistry, 2018, 117: 56−67. DOI: 10.1016/j.soilbio.2017.11.003
[73] Gu S H, Wei Z, Shao Z Y, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes[J]. Nature Microbiology, 2020, 5(8): 1002−1010. DOI: 10.1038/s41564-020-0719-8
[74] Xu Z H, Shao J H, Li B, et al. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation[J]. Applied and Environmental Microbiology, 2013, 79(3): 808−815. DOI: 10.1128/AEM.02645-12
[75] Yang R H, Shi Q, Huang T T, et al. The natural pyrazolotriazine peseudoiodinine from pseudomonas mosselii 923 inhibits plant bacterial and fungal pathogens[J]. Nature Communications, 2023, 14(1): 734. DOI: 10.1038/s41467-023-36433-z
[76] 陈玉, 李宇聪, 刘妍, 等. 植物益生木霉−根系互作机制及其信号物质筛选策略[J]. 植物营养与肥料学报, 2023, 29: 1923−1931. Chen Y, Li Y C, Liu Y, et al. Chen Y, Li Y C, Liu Y. Mechanism of beneficial Trichoderma-root interaction and the screening strategy for signals[J]. Journal of Plant Nutrition and Fertilizers, 2023, 29: 1923−1931.
[77] Ribeiro M S, de Paula R G, Voltan A R, et al. Endo-β-1, 3-glucanase (GH16 Family) from Trichoderma harzianum participates in cell wall biogenesis but is not essential for antagonism against plant pathogens[J]. Biomolecules, 2019, 9(12): 781. DOI: 10.3390/biom9120781
[78] Wang B X, Zhang Z Y, Xu F G, et al. Soil bacterium manipulates antifungal weapons by sensing intracellular type IVA secretion system effectors of a competitor[J]. the ISME Journal, 2023, 17(12): 2232−2246. DOI: 10.1038/s41396-023-01533-7
[79] Wu D, Wang W X, Yao Y P, et al. Microbial interactions within beneficial consortia promote soil health[J]. Science of the Total Environment, 2023, 900: 165801. DOI: 10.1016/j.scitotenv.2023.165801
[80] Andric S, Rigolet A, Arias A A, et al. Plant-associated Bacillus mobilizes its secondary metabolites upon perception of the siderophore pyochelin produced by a Pseudomonas competitor[J]. The ISME Journal, 2023, 17(2): 263−275. DOI: 10.1038/s41396-022-01337-1
[81] Feng H C, Fu R X, Luo J Y, et al. Listening to plant’s Esperanto via root exudates: Reprogramming the functional expression of plant growth-promoting rhizobacteria[J]. New Phytologist, 2023, 239(6): 2307−2319. DOI: 10.1111/nph.19086
[82] Wen T, Zhao M L, Yuan J, et al. Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes[J]. Soil Ecology Letters, 2020, 3(1): 42−51.
[83] Tian T, Sun B B, Shi H W, et al. Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization[J]. The ISME Journal, 2021, 15(9): 2723−2737. DOI: 10.1038/s41396-021-00966-2
[84] Wen T, Xie P H, Liu H W, et al. Tapping the rhizosphere metabolites for the prebiotic control of soil-borne bacterial wilt disease[J]. Nature Communications, 2023, 14(1): 4497. DOI: 10.1038/s41467-023-40184-2
[85] Wen T, Ding Z X, Thomashow L S, et al. Deciphering the mechanism of fungal pathogen-induced disease-suppressive soil[J]. New Phytologist, 2023, 238(6): 2634−2650. DOI: 10.1111/nph.18886
[86] Blundell R, Schmidt J E, Igwe A, et al. Organic management promotes natural pest control through altered plant resistance to insects[J]. Nature Plants, 2020, 6(5): 483−491. DOI: 10.1038/s41477-020-0656-9
[87] Berendsen R L, Vismans G, Yu K, et al. Disease-induced assemblage of a plant-beneficial bacterial consortium[J]. The ISME Journal, 2018, 12(6): 1496−1507. DOI: 10.1038/s41396-018-0093-1
[88] Rodriguez P A, Rothballer M, Chowdhury S P, et al. Systems biology of plant-microbiome interactions[J]. Molecular Plant, 2019, 12(6): 804−821. DOI: 10.1016/j.molp.2019.05.006
[89] Zhang H H, Liu Y P, Wu G W, et al. Bacillus velezensis tolerance to the induced oxidative stress in root colonization contributed by the two-component regulatory system sensor ResE[J]. Plant, Cell & Environment, 2021, 44(9): 3094-3102.
[90] Qian J M, Bai Y. Stuck on you: Bacterial-auxin-mediated bacterial colonization of plant roots[J]. Cell Host & Microbe, 2021, 29(10): 1471−1473.
[91] Rebolledo-Prudencio O G, Estrada-Rivera M, Dautt-Castro M, et al. The small RNA-mediated gene silencing machinery is required in Arabidopsis for stimulation of growth, systemic disease resistance, and the suppression of nitrile-specifier, NSP4 gene by Trichoderma atroviride[J]. Plant Journal, 2022, 109(4): 873−890. DOI: 10.1111/tpj.15599
[92] Li M, Pommier T, Yin Y, et al. Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition[J]. The ISME Journal, 2022, 16(3): 868−875.
[93] Li Z F, Bai X L, Jiao S, et al. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance[J]. Microbiome, 2021, 9: 217. DOI: 10.1186/s40168-021-01169-9
[94] Carrión V J, Perez-Jaramillo J, Cordovez V, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome[J]. Science, 2019, 366: 606−612. DOI: 10.1126/science.aaw9285
[95] Snelders N C, Petti G C, van den Berg G C, et al. An ancient antimicrobial protein co-opted by a fungal plant pathogen for in planta mycobiome manipulation[J]. Proceedings of the National Academy of Sciences, 2021, 118(49): e2110968118. DOI: 10.1073/pnas.2110968118
[96] Valle I D, Webster T M, Cheng H Y, et al. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication[J]. Science Advances, 2020, 6(5): aax8254. DOI: 10.1126/sciadv.aax8254
[97] Kim D R, Jeon C W, Cho G, et al. Glutamic acid reshapes the plant microbiota to protect plants against pathogens[J]. Microbiome, 2021, 9: 244. DOI: 10.1186/s40168-021-01186-8
[98] 蔡祖聪, 黄新琦, 赵军. 作物土传病害防控的健康微生物群落构建原理与实践[J]. 土壤学报, 2023, 60(5): 1213−1220. DOI: 10.11766/trxb202306120227 Cai Z C, Huang X Q, Zhao J. Principles and practice of building healthy microbial community to control soil-borne crop disease in intensive agriculture[J]. Acta Pedologica Sinica, 2023, 60(5): 1213−1220. DOI: 10.11766/trxb202306120227
[99] Zhou Y Y, Yang Z, Liu J G, et al. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease[J]. Nature Communications, 2023, 14(1): 8126. DOI: 10.1038/s41467-023-43926-4



 下载:
下载: